This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: We recommend against using Ivermectin for treatment of patients with any severity of COVID-19 (strong recommendation). It has no benefit in reducing mortality, progression to mechanical ventilation and time to negative PCR and does not increase adverse effects.
DATE OF RECOMMENDATION: 06th October 2022
Definition of mild:
- Symptomatic (any acute COVID-19 related symptoms)
- AND respiratory rate <24/min
- WITHOUT pneumonia or hypoxia
Definition of moderate illness:
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
- severe respiratory distress or respiratory rate >30/min
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
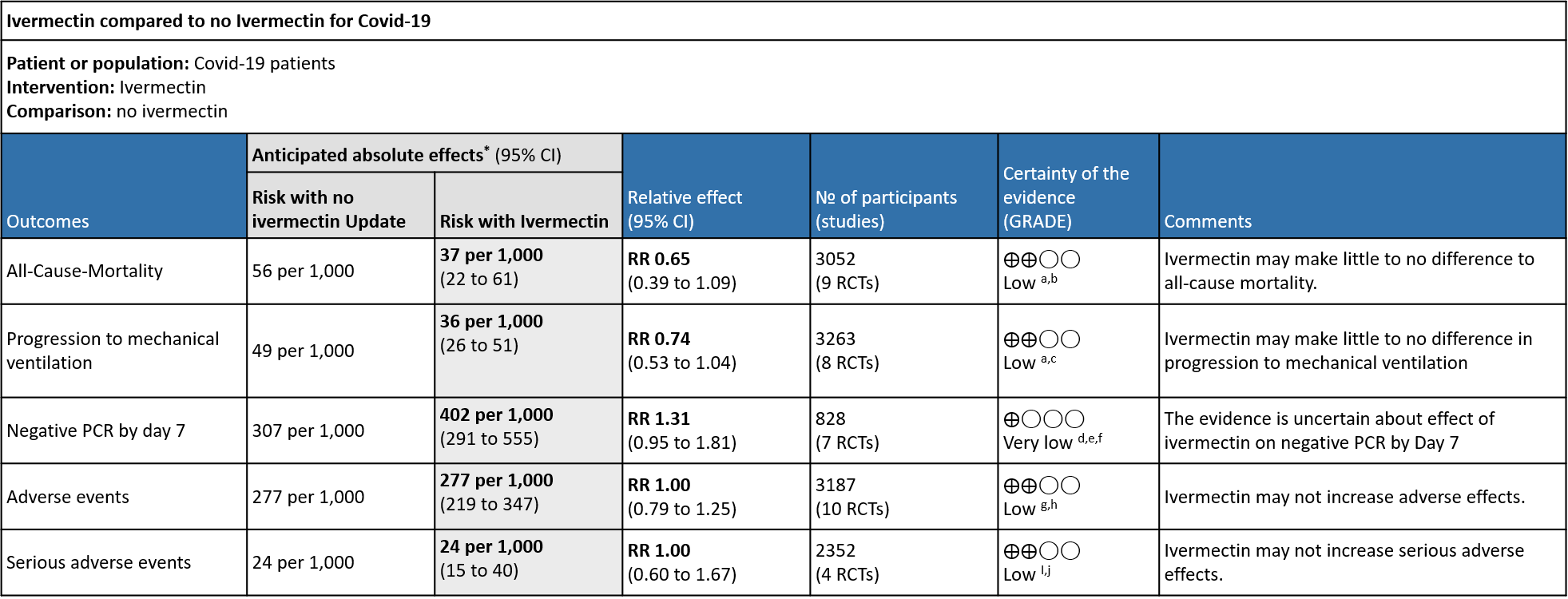
The evidence from 20 randomized controlled trials(1–20), with 4140 participants suggests that Ivermectin does not improve mortality [RR 0.65, 95% CI 0.39, 1.09], progression to mechanical ventilation [RR 0.74, 95% CI 0.53, 1.04] or viral clearance in patients by day 7 with COVID-19 infection [RR 1.31, 95% I 0.95, 1.81]. At the doses used in these trials (median dose is 12 mg once daily orally) it does not have any major adverse events [RR 1.00, 95% CI 0.60, 1.67]. Multiple studies on Ivermectin in Covid-19 have been methodologically unsound with many retracted from peer reviewed literature, further clouding the data(21,22). A stratified analysis of all-cause mortality in studies with low risk of bias showed no benefit with Ivermectin [RR 0.85, 95% CI 0.48, 1.50]
Currently, its use may distract from use of other therapies for which there is better evidence. Indiscriminate use might also reduce its availability for other conditions where its benefit is established, such as parasitic infections. Taking this into account, we recommend against use of ivermectin outside of a randomized controlled trial.
Date of latest search: September 26th 2022
Date of completion & presentation to the Expert Working Group:
Date of planned review:
Evidence Synthesis Team: Harshdeep Acharya, Audrin Lenin,Jane Miracline John, Jisha Sara John, Sherly Shulamite, Richard Kirubakaran, Priscilla Rupali and Bhagteshwar Singh.
Explanations:
a. Downgraded by one level for serious imprecision, due to small absolute number of events, and CIs include important potential benefit and important potential harm.
b. Downgraded by one level for serious risk of bias. Due to Galan 2021 and Niaee 2021 having high risk of bias, and Gonzalez 2021 , Ravikirti 2021 , Abd Elsalam and Shahbaznejad 2021 having some concerns for risk of bias.
c. Downgraded by one level for serious risk of bias, due to Abd Elsalam , Galan 2021, Ravikirti 2021Shahbaznejad and I-tech trial having some concerns for risk of bias.
d. Downgraded by two levels for very serious risk of bias, due to Ahmed 2020 and Bukhari 2021 having high risk of bias and Bieber having some concerns for risk of bias for this outcome.
e. Downgraded by one level for serious inconsistency, due to substantial heterogeneity (I-squared=64%) and visually some trials having point effect estimates very far from those of other trials.
f. Downgraded by one level for serious imprecision, due to CIs overlapping no effect and inability to exclude important benefit.
g. Downgraded by one level for serious risk of bias., due to Chaccour 2021, Chachar 2021, Rocha et. al etc having high risk of bias (accounts for 15% weightage).
h. Downgraded by one level for serious imprecision, due to CIs overlapping including important potential benefit and important potential harm.
i. Downgraded by one level for serious risk of bias, due to Gonzalez 2021 having high risk of bias
j. Downgraded by one level for serious imprecision, due to CIs including important potential benefit and important potential harm.
Ivermectin has been shown to inhibit the replication of SARS CoV2 in vitro; it binds and destabilises the viral protein and prevents it from entering the nucleus (24). However, the drug dosages used in these laboratory studies far exceed those that have been used for other conditions (25). Drug doses and levels required to achieve therapeutic effects in COVID-19 infection in humans based on these studies may be safe, but this has not been studied in clinical trials (26). An additional potential effect may be in modulating the immune system, though this is yet to be studied thoroughly in humans (27).
Although Ivermectin is generally well tolerated, adverse effects like dizziness, tachycardia, postural hypotension, diarrhoea, arthralgia, facial and peripheral oedema have been reported even with single doses as used in parasitic diseases (28). Encephalopathy with permanent disability has been reported when ivermectin has been used in the treatment of onchocerciasis or other parasitic diseases (28). It is predominantly metabolized in the liver (CYP3A4), which may lead to drug-drug interactions.
Due to lack of conclusive evidence from trials, World Health Organization recommends use of ivermectin only in clinical trials (29). Use continues widely, including self-medication, especially in low- and middle-income countries due to easy availability and low cost of the drug (30). Multiple trials on Ivermectin on covid-19 were retracted due to questionable methodology and lack of reliable data. (21,22)
This review aims to provide a summary of the available evidence from randomised clinical trials of ivermectin for treatment of acute COVID-19, for any dose or duration, so the Expert Working Group can provide a recommendation to guide clinicians and researchers regarding the appropriate use of this drug.
We used Cochrane rapid review methods. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 07th June 2022. We also reviewed reference lists of systematic reviews and included studies. We contacted researchers to identify unpublished and ongoing studies.
We included randomized controlled trials (RCTs) testing ivermectin treatment of any dose or duration in people with acute COVID‐19, whether suspected or confirmed. Trials were included if the intervention arm did not combine ivermectin with another experimental drug, and if the comparator arm did not include ivermectin (this could involve use of placebo, standard care, or other potentially active drugs). We excluded trials that did not report any outcomes that could provide usable data for the review, those which were quasi-randomized and those lacking a comparator arm.
We planned to extract data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason) - Duration of hospitalization
- Need for hospitalization (for out-patients)
- Adverse events: all and serious
- Important (secondary):
- Time to clinical improvement
- Time to negative PCR for SARS-CoV-2
- Negative PCR for SARS-CoV-2 by day 7 post-enrolment
- Complications of COVID-19:
- Thrombotic events
- Pulmonary function/fibrosis
- Long covid/ post-covid sequalae
- Secondary infections
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. Data and RoB assessments were checked against a Cochrane systematic review team’s extractions and assessments (we used a consensus approach). In case of any discrepancies, the whole RoB assessment was scrutinised by the whole team for this review, to reach consensus.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to explore heterogeneity in the effect on mortality using subgroup analysis comparing between trials, based on COVID-19 illness severity of participants included and risk of bias. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
We included 20 trials involving 4140 participants, all of whom were adults, and 2122 of whom received ivermectin (1–20). Of these 11 studies are included in the previous recommendation and 9 new studies were added to update the evidence (21,22) While updating the evidence, we excluded 1 study (22) which was present in the previous analysis as it was retracted due to unreliable methodology. There were two trials each from, India, Iran, Pakistan, Brazil, and Mexico; one trial each was reported from Bangladesh, Colombia, Nigeria, Egypt, Israel, Italy, Argentina, Spain, Thailand, and Malaysia. Eleven were in hospitalized patients, four recruited out-patients only, one recruited both, and three did not report care setting. Disease severity, prevalence of comorbidities, and use of co‐interventions varied substantially between trials. The ‘Summary of characteristics of included trials’ table provides further details about the trials.
We found potential risks of bias across all domains; 12 of the 20 trials were at high risk of bias overall for at least one outcome. Risk of bias for each domain per trial is displayed alongside the Forest plots below. Studies excluded at full-text review are listed in the References section, with the reason provided in brackets (21,22,31–40).
The following comparisons were investigated in the trials. We compared outcomes for arms randomised to ivermectin vs. outcomes in arms with placebo, standard care, or agents considered inactive or ineffective against COVID-19. Where multiple arms contained ivermectin without another experimental agent, we combined results in those arms into a single ivermectin arm, but we did not double-count controls. Where another experimental agent undergoing investigation was combined with ivermectin, that trial arm was excluded from the analysis (e.g., doxycycline).
- Ten trials (3–5,8,9,11,14,17,18,20) compared ivermectin vs. placebo (2358 participants)
- Six [18;22-23;25-26;29] compared ivermectin vs. standard care (1244 participants)
- One (1) compared ivermectin vs. placebo vs. a combination of ivermectin & doxycycline in three arms (72 participants; 24 participants in ivermectin & doxycycline arm excluded)
- One (5) compared ivermectin vs. placebo vs. hydroxychloroquine in three arms (106 participants)
- One (2) compared ivermectin vs. lopinavir/ritonavir (62 participants)
- One (4) compared ivermectin vs. chloroquine or hydroxychloroquine (168 participants)
All-Cause-Mortality: Low certainty evidence from 9 RCTs (6,8,10,12,15,19,20) with 3052 participants shows that ivermectin may result in little to no difference in mortality [RR 0.65, 95%CI 0.39,1.09]. As the studies have some concerns and high risk of biases, a stratified analysis of the data was done based on ‘Risk of bias’. The trials (8,20) with Low risk of bias showed no difference in mortality in patients receiving Ivermectin vs no Ivermectin [RR 0.85, 95%CI 0.48,1.50]
Progression to mechanical ventilation: Low certainty evidence from 8 trials (6,11,12,15,16) with 3263 participants showed that Ivermectin may have little to no effect in the progression to mechanical ventilation [RR 0.74, 95%CI 0.53,1.04]. 5 of the 8 included studies have some concerns.
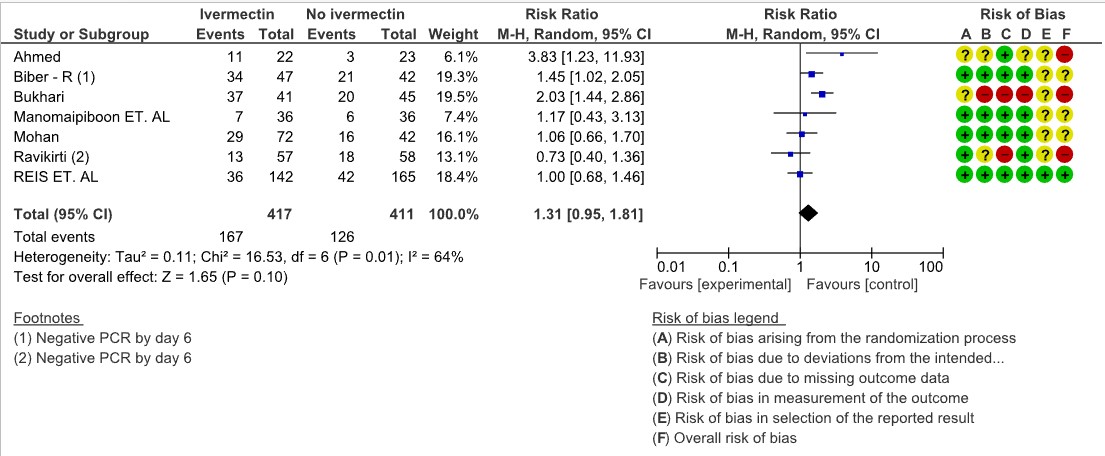
Negative PCR by day 7: Very low certainty evidence from 7 trials (1,3,11,13,17,20) with 828 participants for viral clearance at day 7 [RR 1.31, 95%CI 0.95,1.81] gives no impression of Ivermectin’s effect on viral clearance at Day 7. Of the 7 trials, 3 of them where of High Risk of bias, 3 of them had some concerns and only 1 trial with low risk of bias and had only 19% weightage. (Look at the forest plot).
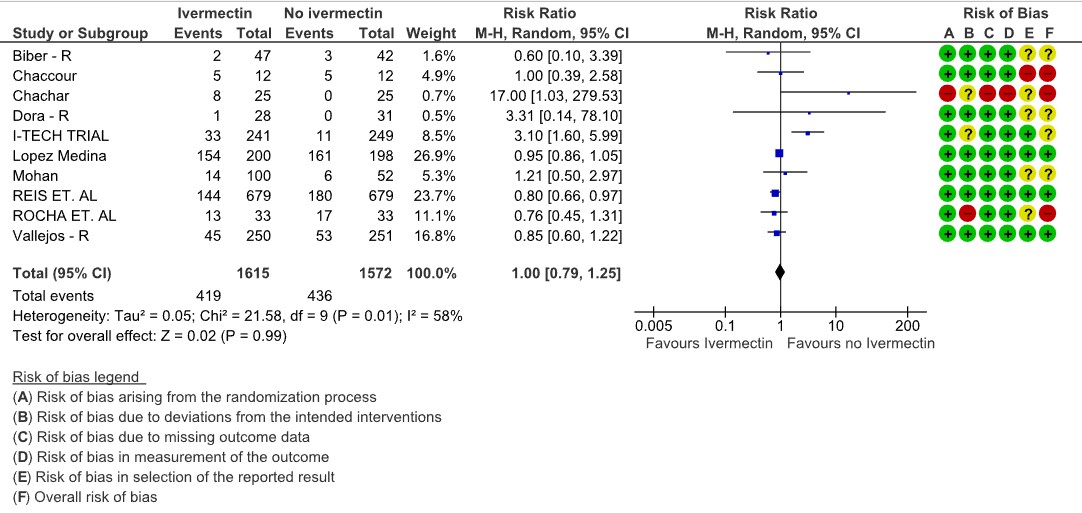
Adverse events: Low certainty evidence from 10 RCTs (4,5,8,9,13,14,16,18–20) with 3187 participants shows that Ivermectin results in no significant adverse events RR 1.00, [95%CI 0.79,1.25].
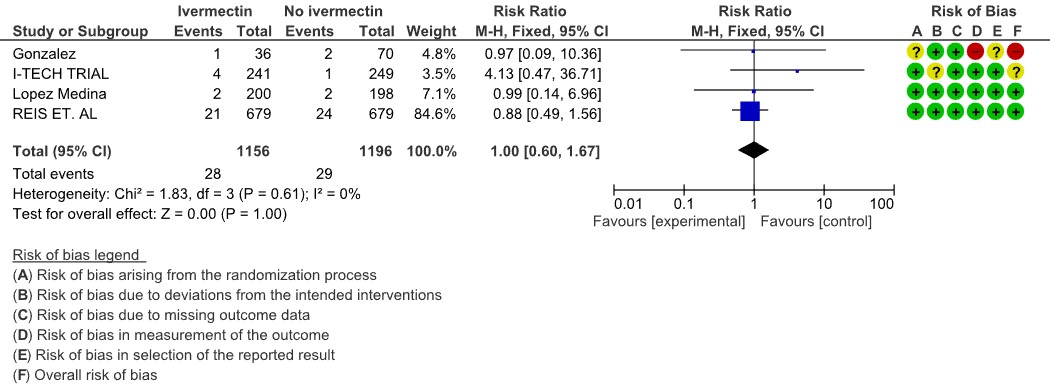
Serious adverse events: Low certainty evidence from 4 RCTs (7,8,19,20) with 2352 participants shows that Ivermectin results in no significant serious adverse events [RR 1.00, 95%CI 0.60,1.67].
One trial reported a higher risk of discontinuation of ivermectin vs. placebo due to an adverse event (RR 2.97; 95% CI 1.10 to 8.02; 1 trial (8); 398 participants).
95% confidence intervals for pooled effect estimates for all of the following outcomes not included in the summary of findings table included potential benefit and potential harm from ivermectin: need for critical or intensive care (2 trials (6,11); 283 participants); discharge from hospital by day 10 post-enrolment (1 trial (11); 115 participants); deterioration by 2 points on 8-point clinical ordinal scale (1 trial (8); 398 participants); lack of fever on day 7 (1 trial (1); 36 participants); lack of symptoms on day 7 (1 trial (5); 50 participants); and thrombotic events (1 trial (7); 106 participants). We were unable to pool data for time to clinical improvement as they were not reported in a way that was amenable to meta-analysis (3 trials (4,8,9); 149 participants).
No comparative data could be extracted for risk of progression to oxygen therapy; need for hospitalisation in outpatients; or post-acute COVID-19 pulmonary function/fibrosis or other sequelae; or secondary infections.
Lack of uniform criteria for COVID-19 severity, substantial overlap, and lack of clear reporting of severity in the included trials prevented a meaningful subgroup analysis by severity.
Furthermore, a lack of within trial comparison prevented subgroup analysis by age, duration of symptoms or dose of ivermectin. In addition, data of safety and efficacy in specific subgroups such as pregnancy, children, liver, and kidney disease were not available in the trials included in the rapid review.
CQ: Chloroquine; ECOG: Eastern Cooperative Oncology Group score; HCQ: Hydroxychloroquine; Lpv/Rtn: Lopinavir/Ritonavir; NEWS: National early warning score; RCT: Randomized control trial; RTPCR: Reverse transcription polymerase chain reaction; SoC: Standard of care
1. Mortality, stratified by risk of bias
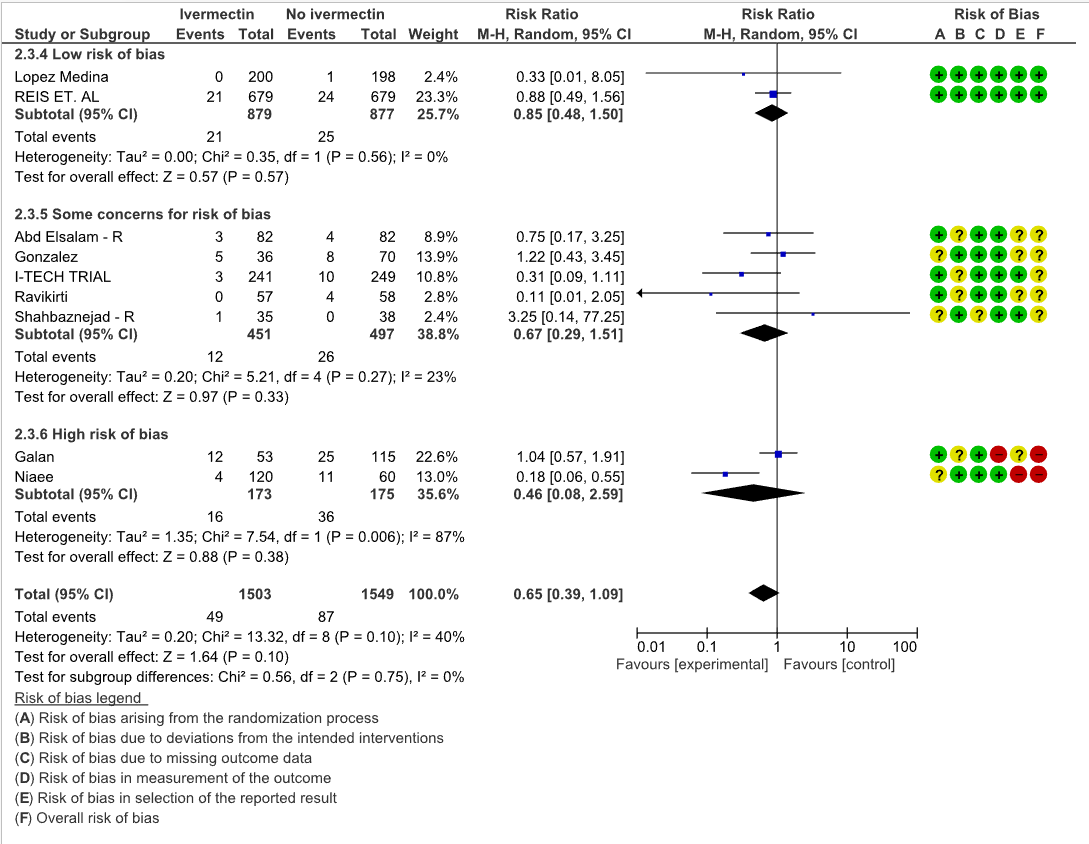
2. Progression to mechanical ventilation
3. Negative PCR for SARS-CoV-2 by day 7
4. Adverse events
5. Serious adverse events
The Antiviral Expert Working Group met on 13th June 2021 to consider Favipiravir as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Favipiravir.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India with more than 44 million cases and over 0.53 million deaths has significantly impacted and stressed the health structure of the country. During the inception of the pandemic,with a shortage of intensive care unit beds, oxygen and trained personnel the country was facing a major health crisis. This had prompted many irrational and experimental treatment interventions like Favipiravir in all severity of patients across the country in patients hospitalized in COVID-19 without clear indication or evidence in which subgroup of population or disease severity the drug is effective for. Currently the drug is not used popularly for the treatment of covid-19. The group judged the problem to be of utmost priority.
Desirable effects
The panel agreed that the evidence suggests that Favipiravir doesn’t have a clinically significant effect on mortality, time to negative PCR, reducing progression of disease, or preventing critical or intensive care admissions. There is no significant effect in reducing the duration of hosptalisatin , time to clinical improvement or time to viral clearance.
Undesirable effects
The pooled data did not suggest any increase in serious or other adverse events, or adverse events leading to discontinuation when Favipiravir was added to usual care or with active comparators. However, the panel felt that hyperuricemia is a well-known adverse effect of favipiravir which is self-limiting in most cases and caution should be advised for use in renal failure and gout.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence as very low for all-cause mortality, duration of hospitalization, progression to respiratory failure, progression to oxygen therapy, progression to invasive mechanical ventilation, any adverse events and serious adverse events. The certainty of evidence was high for duration of hospitalization and moderate for adverse events-hyperuricemia. The expert working group agreed with these judgements and rated the overall certainty as very low.
Values
The EWG felt that all the outcomes including those of mortality, progression to respiratory failure, oxygen therapy or mechanical ventilation, critical or intensive care for any reason, duration of hospitalisation and outcomes related to adverse events were expressed variably in the studies. However, there is probably no important uncertainty or variability on how people would value the main outcomes.
Balance of effects
The expert working group felt that the balance of effects does not favor either the intervention or the comparison. The panel felt that there is no convincing evidence to prove the benefit of this intervention, since there were different comparators and varying severity of disease across all studies. The intervention was also found to be not efficacious as per the sensitivity analysis where results were similar when we compared after splitting into 2 categories -SOC + active comparator (another antiviral) vs together.
Resources required
The group felt that the costs were small. The erratic supply chain had led to an increased demand for this drug during the initial phase of the second wave of the pandemic despite its uncertain clinical benefits until the drug was removed from the ministry of health guidelines for COVID-19 on June 7th 2021. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The panel discussed that even though there was no research evidence that evaluated cost of Favipiravir in an Indian context, it favors the comparison, as this intervention was not of clinical benefit and conferred a cost for implementation and would always be in addition to the standard care.
Equity
At this point in time this intervention would increase equity if found efficacious as it would prevent admission into hospital. However as of now due to the lack of efficacy unnecessary adding of this drug would incur an additional cost and hence reduce equity.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policymakers, patients and clinicians) if efficacious as it is an oral drug that is probably quite safe, but taking evidence and cost into account a well-informed clinician would be unlikely to use it.
Feasibility
This is a feasible intervention if found efficacious as it is easy to deliver and available easily over the counter in the country.
Currently the evidence to support using Favipiravir for the treatment of COVID-19 in any patient group is lacking, as the certainty of the evidence to date is very low. Regarding adverse effects, there is evidence to suggest that there is definite hyperuricemia when given as a treatment for COVID-19 and the benefits (or the lack thereof) right now do not outweigh the risk. It is contraindicated in pregnancy based on data from animal studies showing teratogenicity thus caution should be exercised when prescribing for patients of reproductive age. Favipiravir is an oral drug, making it suitable as an outpatient or inpatient treatment. The dose is 1800 mg twice daily on the first day, followed by 800mg twice daily up to day 14. Previously there were only 200mg and 400mg doses available making the pill burden quite high which might affect treatment adherence, however 800mg tablets are now available. A two week treatment course costs around Rs.3600. If further evidence emerges showing Favipiravir is effective and safe for the treatment of COVID-19, it should be straightforward to implement this treatment into existing protocols.
Our conditional recommendation against the use of Favipiravir applies to all subgroups of patients with COVID-19. The group considered all trials of Favipiravir and found no subgroups where there was benefit in clinically meaningful endpoints whether evaluated by category of severity or by co-morbidities. There are very limited data available assessing its use in patients with liver or kidney disease.
Although the evidence for discontinuation of the drug because of the undesirable effects reported with widespread use such as hyperuricemia, liver dysfunction and chest pain has so far been low, this needs careful monitoring as the potential for drug-related harm cannot be ruled out.
There is currently no evidence to support the use of Favipiravir in any patient group for COVID-19 treatment. There is a need for conduct of well-structured, adequately powered randomized controlled trials with a low risk of bias to address the following:
- Does use of Favipiravir in different subgroups of disease severity or in different special populations (children, immunosuppressed, co-morbid conditions) keep patients from getting admitted into hospital?
- What dose of Favipiravir is safe and efficacious for the treatment of COVID-19?
- Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis [Internet]. 2021 Feb 1 [cited 2022 Sep 30];103:214–6. Available from: https://www.sciencedirect.com/science/article/pii/S1201971220325066
- Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos. QJM Int J Med [Internet]. 2021 Nov 1 [cited 2022 Sep 30];114(11):780–8. Available from: https://doi.org/10.1093/qjmed/hcab035
- Bukhari KHS, Asghar A, Perveen N, Hayat A, Mangat SA, Butt KR, et al. Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease [Internet]. medRxiv; 2021 [cited 2022 Sep 30]. p. 2021.02.02.21250840. Available from: https://www.medrxiv.org/content/10.1101/2021.02.02.21250840v1
- Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine [Internet]. 2021 Feb 1 [cited 2022 Sep 30];32:100720. Available from: https://www.sciencedirect.com/science/article/pii/S2589537020304648
- Chachar AZK, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients. Int J Sci [Internet]. 2020 Oct 9 [cited 2022 Sep 30];9(09):31–5. Available from: https://www.ijsciences.com/pub/article/2378
- Galan LEB, Santos NM dos, Asato MS, Araújo JV, de Lima Moreira A, Araújo AMM, et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog Glob Health [Internet]. 2021 May 19 [cited 2022 Sep 30];115(4):235–42. Available from: https://doi.org/10.1080/20477724.2021.1890887
- Gonzalez JLB, Gámez MG, Enciso EAM, Maldonado RJE, Palacios DH, Campos SD, et al. Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial [Internet]. medRxiv; 2021 [cited 2022 Sep 30]. p. 2021.02.18.21252037. Available from: https://www.medrxiv.org/content/10.1101/2021.02.18.21252037v1
- López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA [Internet]. 2021 Apr 13 [cited 2022 Sep 30];325(14):1426–35. Available from: https://doi.org/10.1001/jama.2021.3071
- Mohan A, Tiwari P, Suri T, Mittal S, Patel A, Jain A. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. Res Sq [Internet]. 2021 Feb 2 [cited 2022 Sep 30]; Available from: https://www.researchsquare.com
- Niaee MS, Gheibi N, Namdar P, Allami A, Zolghadr L. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multi-center clinical trial. Res Sq [Internet]. 2020 Nov 24 [cited 2022 Sep 30]; Available from: https://www.researchsquare.com
- Kirti R, Roy R, Pattadar C, Raj R. Ivermectin as a potential treatment for mild to moderate COVID-19: A double blind randomized placebo-controlled trial. Semantic Sch. 2021 Jan 9;
- Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study. J Med Virol [Internet]. 2021 [cited 2022 May 9];93(10):5833–8. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.27122
- Biber A, Mandelboim M, Harmelin G, Lev D, Ram L, Shaham A, et al. Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19 – A double-blind, randomized placebo-controlled trial [Internet]. medRxiv; 2021 [cited 2022 Sep 30]. p. 2021.05.31.21258081. Available from: https://www.medrxiv.org/content/10.1101/2021.05.31.21258081v1
- Buonfrate D, Chesini F, Martini D, Roncaglioni MC, Ojeda Fernandez ML, Alvisi MF, et al. High Dose Ivermectin for the Early Treatment of COVID-19 (COVER Study): A Randomised, Double-Blind, Multicentre, Phase II, Dose-Finding, Proof of Concept Clinical Trial [Internet]. Rochester, NY; 2021 [cited 2022 Sep 30]. Available from: https://papers.ssrn.com/abstract=3918289
- Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, et al. Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-blind, Randomized, Controlled Clinical Trial. Clin Ther. 2021 Jun;43(6):1007–19.
- Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021 Jul 2;21(1):635.
- Manomaipiboon A, Pholtawornkulchai K, Pupipatpab S, Suraamornkul S. Efficacy and safety of ivermectin in the treatment of mild-to-moderate COVID-19 infection: A randomized, double blind, placebo, controlled trial | Research Square. Res Sq [Internet]. 2022 Feb 2 [cited 2022 Sep 30]; Available from: https://www.researchsquare.com/article/rs-1290999/v1
- Rocha C de la, Cid-Lopez MA, Venegas-Lopez BI, Gómez-Mendez SC, Sánchez-Ortiz A, Pérez-Ríos AM. Ivermectin compared with placebo in the clinical evolution of Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial. Res Sq [Internet]. 2022 May 9; Available from: https://www.researchsquare.com/article/rs-1640339/v1
- Lim SCL, Hor CP, Tay KH, Mat Jelani A, Tan WH, Ker HB, et al. Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern Med [Internet]. 2022 Apr 1 [cited 2022 Jul 29];182(4):426–35. Available from: https://doi.org/10.1001/jamainternmed.2022.0189
- Reis G, Silva EASM, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med [Internet]. 2022 May 5 [cited 2022 Aug 29];386(18):1721–31. Available from: https://doi.org/10.1056/NEJMoa2115869
- Elgazzar A, Eltaweel A, Youssef SA, Hany B, Hafez M, Moussa H. Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic. Res Sq [Internet]. 2020 Dec 28 [cited 2022 Sep 30]; Available from: https://www.researchsquare.com
- Pott-Junior H, Paoliello MMB, Miguel A de QC, da Cunha AF, de Melo Freire CC, Neves FF, et al. RETRACTED: Use of ivermectin in the treatment of Covid-19: A pilot trial. Toxicol Rep [Internet]. 2021 Jan 1 [cited 2022 Sep 30];8:505–10. Available from: https://www.sciencedirect.com/science/article/pii/S2214750021000445
- Popp M, Stegemann M, Metzendorf MI, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID‐19. Cochrane Database Syst Rev [Internet]. 2021 [cited 2022 Sep 30];(4). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD015017/full
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res [Internet]. 2020 Jun 1 [cited 2022 Sep 30];178:104787. Available from: https://www.sciencedirect.com/science/article/pii/S0166354220302011
- Schmith VD, Zhou J (Jessie), Lohmer LRL. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID‐19. Clin Pharmacol Ther [Internet]. 2020 Oct [cited 2022 Sep 30];108(4):762–5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7267287/
- Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002 Oct;42(10):1122–33.
- Zhang X, Song Y, Ci X, An N, Ju Y, Li H, et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res [Internet]. 2008 Nov 1 [cited 2022 Sep 30];57(11):524–9. Available from: https://doi.org/10.1007/s00011-008-8007-8
- Chandler RE. Serious Neurological Adverse Events after Ivermectin—Do They Occur beyond the Indication of Onchocerciasis? Am J Trop Med Hyg [Internet]. 2017 Dec 4 [cited 2022 Sep 30];98(2):382–8. Available from: https://www.ajtmh.org/view/journals/tpmd/98/2/article-p382.xml
- Therapeutics and COVID-19: living guideline [Internet]. [cited 2022 Feb 11]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1
- Molento MB. COVID-19 and the rush for self-medication and self-dosing with ivermectin: A word of caution. One Health Amst Neth. 2020 Dec;10:100148.
- Podder CS, Chowdhury N, Sina MI, Haque WMMU. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC J Med Sci [Internet]. 2021 Apr 5 [cited 2022 Sep 30];14(2):11–8. Available from: http://www.imcjms.com/registration/journal_full_text/353
- Shoumann WM, Hegazy AA, Nafae RM, Ragab MI, Samra SR. Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial. JCDR [Internet]. 2021 Feb [cited 2022 Sep 30];15(2):OC27–32. Available from: https://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2021&volume=15&issue=2&page=OC27&issn=0973-709x&id=14529
- Rezai M. IRCT | Effectiveness of Ivermectin in the Treatment of Coronavirus Infection in Patients admitted to Educational Hospitals of Mazandaran in 2020 [Internet]. [cited 2022 Sep 30]. Available from: https://en.irct.ir/trial/49174
- Fawaz M, RaaD H. In vivo use of ivermectin (IVR) for treatment for corona virus infected patients (COVID-19): a randomized controlled trial [Internet]. [cited 2022 Sep 30]. Available from: http://www.chictr.org.cn/showproj.aspx?proj=54707
- Chahla RE, Ruiz LM, Ortega ES, Morales MF, Barreiro F, George A, et al. A Randomized Trial - Intensive Treatment Based in Ivermectin and Iota-Carrageenan as Pre-Exposure Prophylaxis for Covid- 19 in Healthcare Agents [Internet]. medRxiv; 2021 [cited 2022 Sep 30]. p. 2021.03.26.21254398. Available from: https://www.medrxiv.org/content/10.1101/2021.03.26.21254398v1
- Chowdhury ATMM, Shahbaz M, Karim MR. A Randomized Trial of Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin therapy on COVID19 patients. 2020 Jul 14 [cited 2022 Sep 30]; Available from: https://www.researchsquare.com
- Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq [Internet]. medRxiv; 2020 [cited 2022 Sep 30]. p. 2020.10.26.20219345. Available from: https://www.medrxiv.org/content/10.1101/2020.10.26.20219345v1
- Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Antiviral Effect of High-Dose Ivermectin in Adults with COVID-19: A Pilot Randomised, Controlled, Open Label, Multicentre Trial [Internet]. Rochester, NY; 2020 [cited 2022 Sep 30]. Available from: https://papers.ssrn.com/abstract=3714649
- Okumuş N, Demirtürk N, ÇETİNKAYA RA, GÜNER R, Avcı İY, ORHAN S. Evaluation of the Effectiveness and Safety of Adding Ivermectin to Treatment in Severe COVID-19 Patients. Res Sq [Internet]. 2021 Feb 24 [cited 2022 Sep 30]; Available from: https://www.researchsquare.com
- Mahmud R, Rahman MM, Alam I, Ahmed KGU, Kabir AKMH, Sayeed SKJB, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021 May;49(5):3000605211013550.
- AIIMS C. Clinical guidance for Management of Adult COVID-19 Patients [Internet]. AIIMS NEW. [cited 2022 Sep 30]. Available from: https://aiims.edu/en/notices/notices.html?id=11611
Covid Management Guidelines India Group - Anti-viral Working Group. Ivermectin. Covid Guidelines India; Published online on Oct 06th, 2022; URL: https://indiacovidguidelines.org/ivermectin-2/ (date<>).
