This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: We do not recommend tocilizumab for non-severe COVID-19. We suggest a single dose of tocilizumab (8mg/kg, maximum 800mg) to be added to corticosteroids for people hospitalized with severe or critical COVID-19 with rapidly progressive respiratory failure and significant systemic inflammation (conditional recommendation).
Note: significant systemic inflammation is usually characterized by clinical features such as high fever and elevated inflammatory markers, such as C-reactive protein of greater than 100mg/L (10mg/dL).
DATE OF RECOMMENDATION: 04th October 2022
Definition of mild:
- Symptomatic (any acute COVID-19 related symptoms)
- AND respiratory rate <24/min
- WITHOUT pneumonia or hypoxia
Definition of moderate illness:
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
- Severe respiratory distress or respiratory rate >30/min
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
The evidence from 22 RCTs indicates that tocilizumab probably reduces mortality by 12% [RR 0.88, 95% CI 0.81,0.94] in severe to critically ill Covid-19 patients. It also reduces progression to ventilation, ECMO or death in patients with severe or early critical COVID-19 [RR 0.85, 95% CI 0.78,0.92] with significant systemic inflammation and severe hypoxia. In the studies examined, most participants (>65%) were receiving steroids, had an average C-reactive protein of greater than 100 mg/L (10mg/dL), and needed oxygen therapy. One of the larger trials mandated initiation within 24 hours of intensive care admission. In increases clinical improvement at 28 days and may reduce time to clinical improvement. The effects are modest.
The evidence was very uncertain for Tocilizumab causing adverse events [RR 1.11, 95% CI 0.95,1.29] and serious adverse events [0.94, 95% CI 0.82, 1.07], but the effect estimates did not suggest an increase in the arms receiving tocilizumab. Tocilizumab has a well-defined safety profile and is widely used for other conditions.
There is a lack of evidence for the use of tocilizumab in patients with non-severe COVID-19. Only 4 studies (1) were available and included mild-moderately ill patients without optimal information.
Therefore, the panel judged that the evidence for modest benefit outweighs risk of harm in a narrowly defined group of patients, hospitalized with severe or critical COVID-19, with evidence of significant systemic inflammation, and respiratory failure worsening despite use of systemic corticosteroid therapy.
Date of latest search: 15th October 2021
Date of completion of Summary of findings table and presentation to Expert Working Group: April 5, 2022
Evidence synthesis team: Dr. Anju Susan Jacob, Dr. Amita Jacob, Dr. Jane Miracline, Dr. Bhagteshwar Singh, Mr. Richard Kirubakaran, Dr. Priscilla Rupali.
According to World Health Organization, there have been 601,189,435 confirmed cases of COVID-19, including 6,475,346 deaths, reported to WHO, by September 2, 2022 (2) Systemic Inflammatory markers like Ferritin, IL-6, D-dimer are markedly elevated in severe Covid-19 patients. A meta-analysis of 6 studies showed that in complicated Covid-19 the marked elevation in the Interleukin-6 (IL-6) is significantly associated with ARDS and death (3) Tocilizumab, an IL-6 inhibiting monoclonal antibody, inhibits the inflammatory pathway via IL-6 and reduces the cytokine storm and other inflammatory responses in a severe to critical Covid-19 patient. Tocilizumab is used in the treatment of autoimmune diseases like rheumatoid arthritis and systemic sclerosis (4)
The updating of the evidence summary of the guideline for the Tocilizumab in Covid-19 focuses on evidence regarding the efficacy and safety of Tocilizumab in various severity of Covid-19 patients. The 2021 recommendations were recommending a conditional use of Tocilizumab in Severe to Critical Covid-19 patients.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 15th October 2021. We included randomized controlled trials (RCTs) testing Remdesivir in people with COVID‐19, and extracted data for the following pre-defined outcomes:
Critical (primary for this review):
- All-cause mortality
- Time to clinical recovery
- Non-invasive or mechanical ventilation
Important (secondary):
Secondary outcomes
- Admission to critical care
- Time to and duration of ventilation
- Length of critical care & hospital stay
- Organ supports other than ventilation
- Change in CRP at 48h (in %)
Adverse events
- All and Serious adverse events
- Nosocomial/opportunistic infections
- GI perforation/ Appendicitis
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. Data and RoB assessments were checked by the second reviewer. The entire RoB assessment was scrutinized by the whole team for this review, to reach consensus.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and hazard ratios for continuous outcomes, with 95% confidence intervals (CIs). We performed a subgroup analysis to explore the effect on mortality stratified by different oxygen and ventilation requirements at baseline.
We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
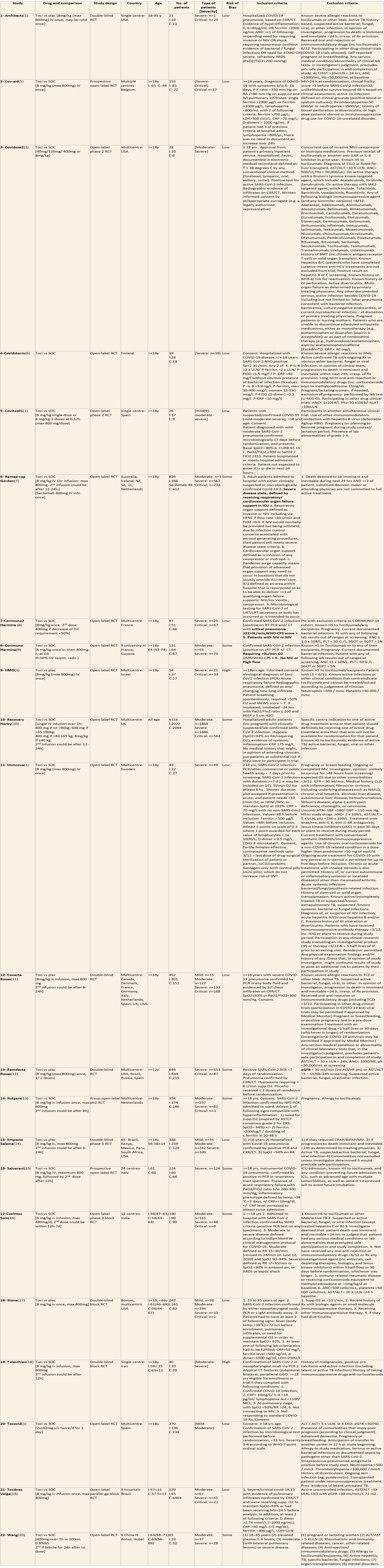
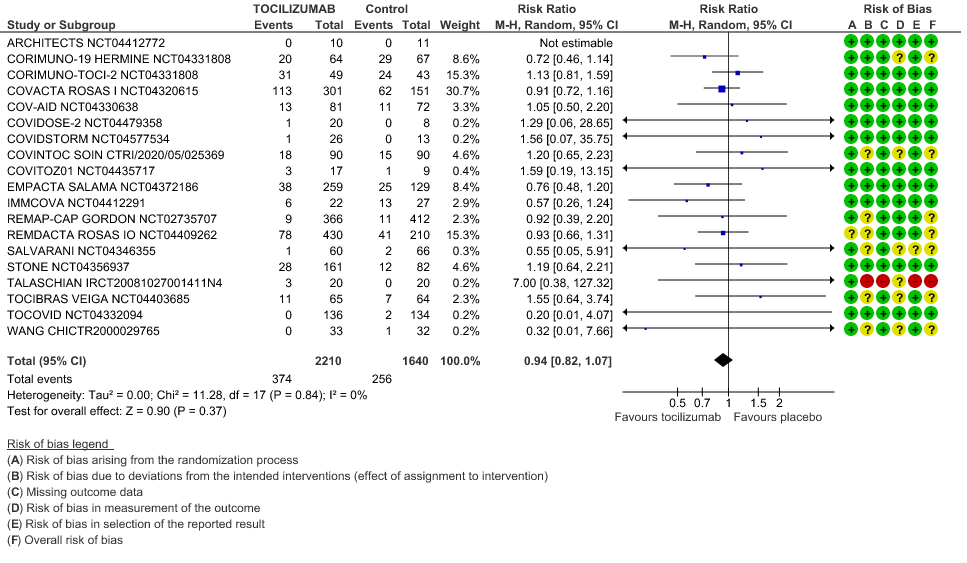
We included 22 trials involving 8436 adult participants, in which 4449 received Tocilizumab. Three studies were reported from US, 2 studies from Spain, and 5 studies were conducted in multiple nations. Rest of the 12 studies reported 1 each from Belgium, Brazil, China, Finland, France, India, Iran, Israel, Italy, Netherlands, Sweden and UK. Of the included trials only 4 trials included mild-moderate patients. Rest of the studies included severe to critically ill participants. Co-interventions varied among different trials, but 65% of the trial participants received glucocorticoids and 20% of the participants received Remdesivir as a co-intervention. The risk of Bias assessment was done among the trials and 11 studies were found to be of low risk of bias, out of 22, twelve studies (1,9–15) were found to have low risk of bias, 8 studies (9,16–22) have some concerns and 2 studies (1,23) were of High RoB.
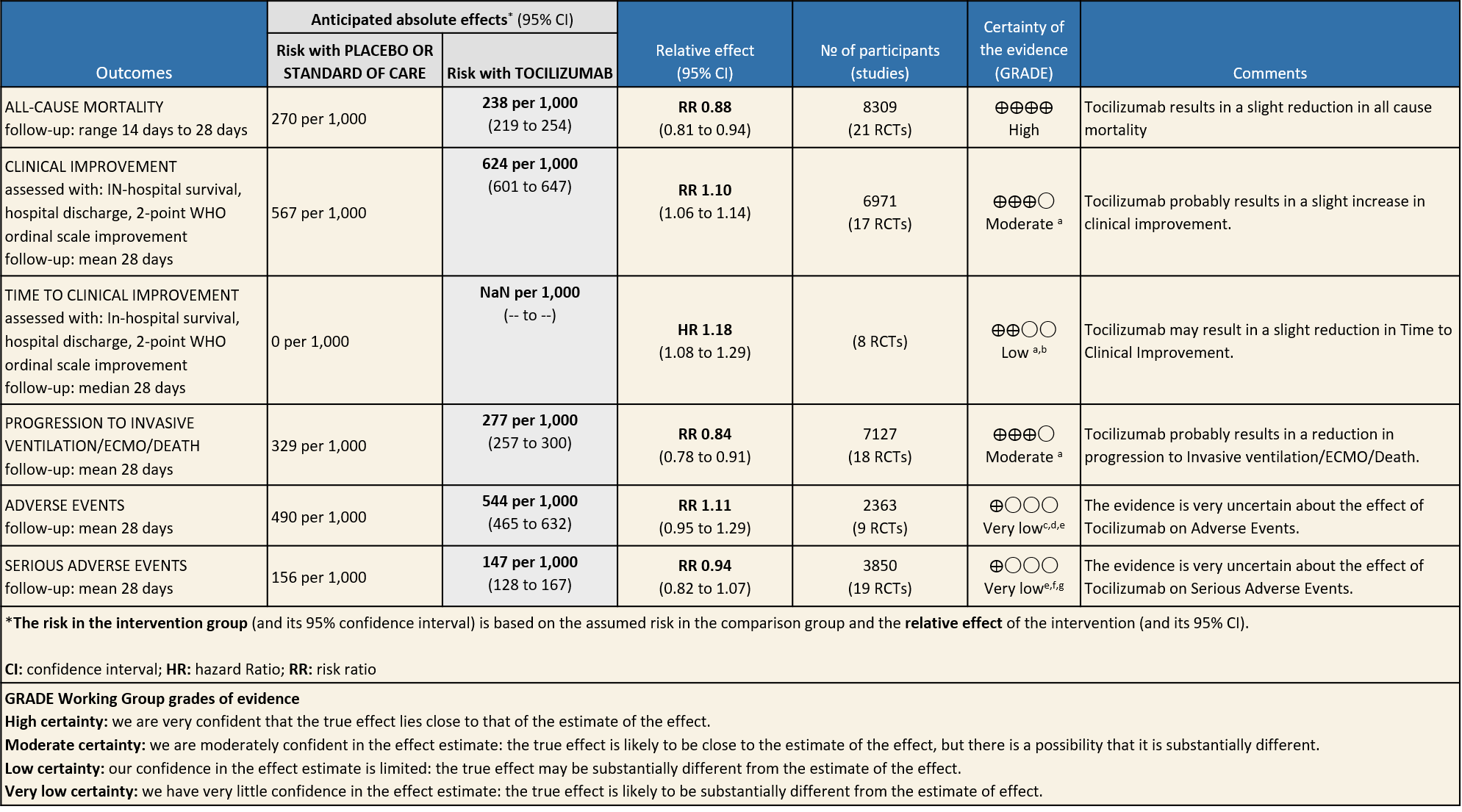
All-Cause Mortality: High certainty of evidence from 21 trials (1,9–26) including 8309 participants found that Tocilizumab reduces mortality in severe to critically ill COVID-19 patients [RR 0.88, 95% CI 0.81, 0.94].
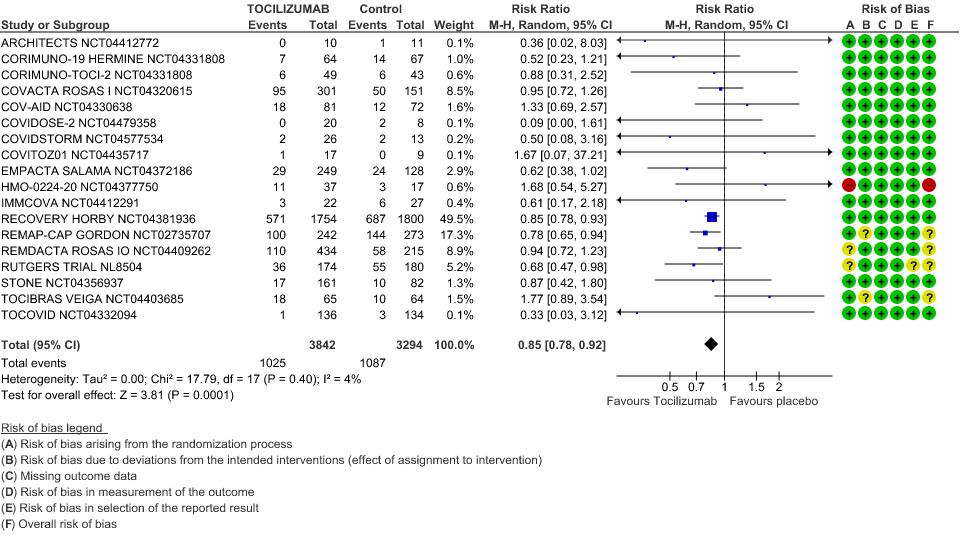
Progression to Invasive Ventilation/ECMO/Death: Moderate certainty of evidence from 18 RCTs (1,9–15,17–19,21,25) including 7127 participants found that Tocilizumab probably results in a reduction in the progression to Ventilation/ECMO/Death [RR 0.85, 95% CI 0.78,0.92].
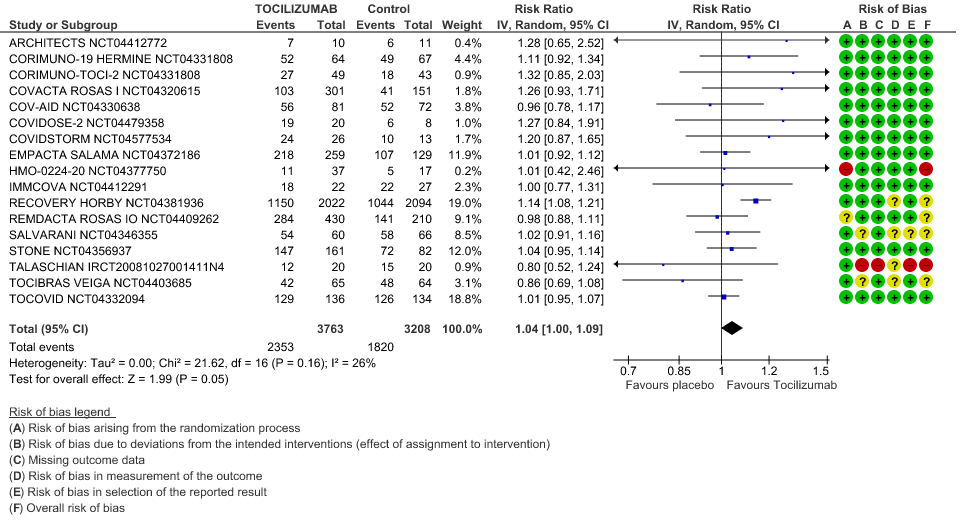
Clinical improvement: Moderate certainty of evidence from 17 RCTs (1,9–15,18,20,21,23,25) including 6971 participants, showed that Tocilizumab probably results in a slight increase in clinical improvement in severe to critically ill Covid 19 patients [RR 1.04, 95% CI 1.00, 1.09]
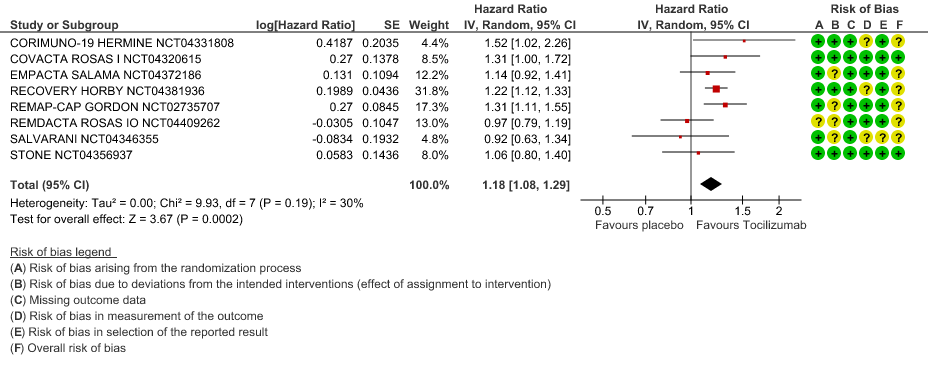
Time to Clinical improvement: Low certainty of evidence from 8 RCTs (9,10,13–15,17,18,20) showed, that treating with Tocilizumab may result in slight reduction for the time to clinical improvement [HR 1.18, 95% CI 1.08,1.29]
Adverse events: Very low certainty of evidence from 9 RCTs (10,13,15,16,18,20–22,25) with 2363 participants are very uncertain about the effects of Tocilizumab in serious adverse effects [RR 1.11 95% CI 0.95, 1.29].
Serious Adverse Events: Very low certainty of evidences from 19 RCTs (1,9–13,15–18,20–23,25) with 3850 participants is very uncertain about the effects of Tocilizumab in Serious Adverse events [RR 0.94, 95% CI 0.82, 1.07].
1. All-Cause Mortality
2. Progression to Invasive Ventilation/ECMO/Death
3. Clinical Improvement
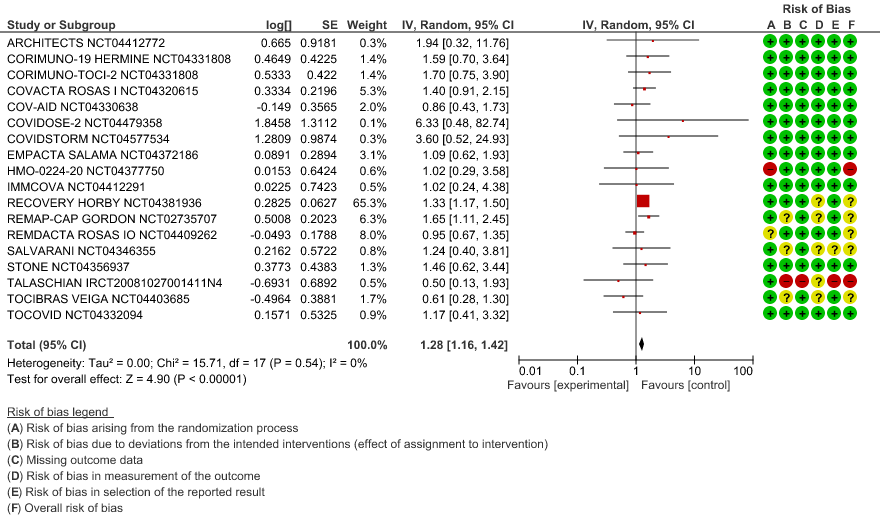
When event rate [common event] >20% OR may overestimate. Hence, we calculated RR with 95% CI – 1.14 [1.08, 1.18]
4. Time to Clinical Improvement
5. Adverse Events
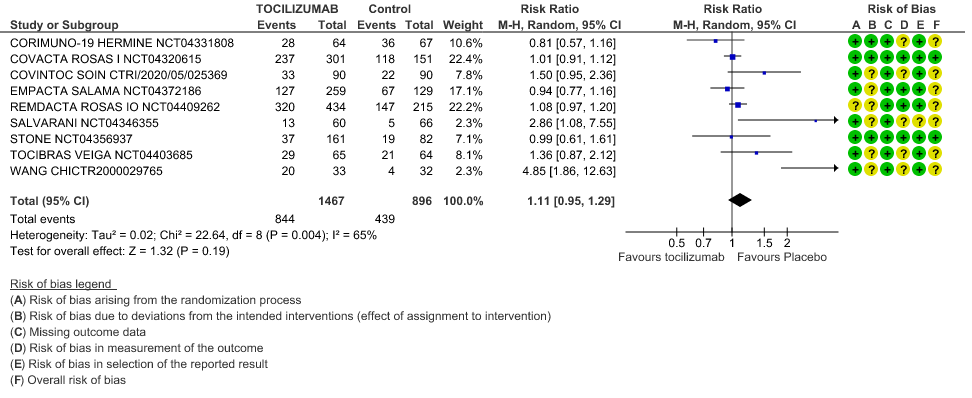
6. Serious Adverse Events
Problem
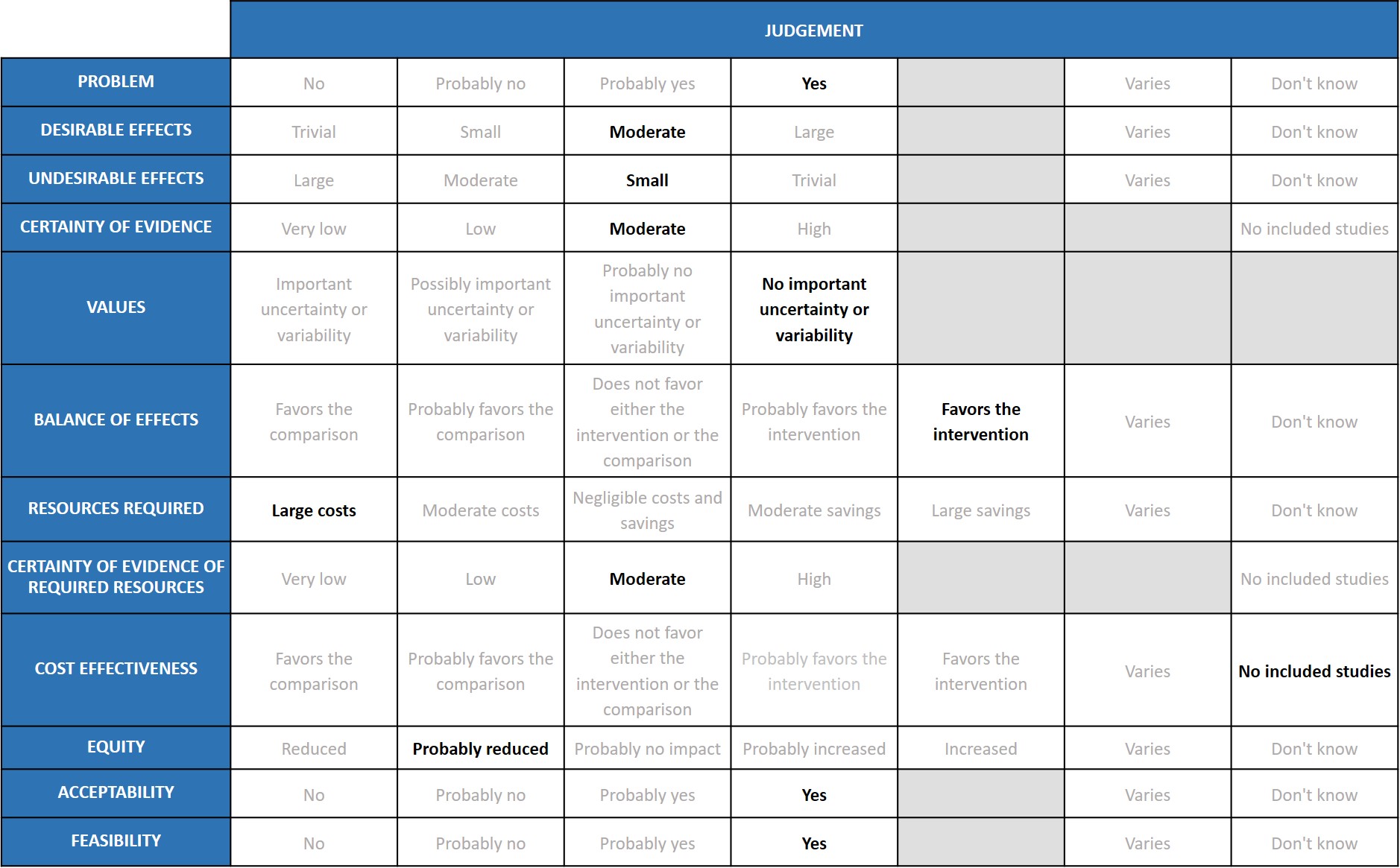
COVID-19 infection is still a problem in India. But the new Omicron variant is not causing severe disease, compared to Delta variant which caused severe disease and mortality. Tocilizumab seems to be beneficial in severe to critical disease where cytokine release syndrome is significant.
Desirable effects
Tocilizumab reduces mortality on average by 12% (95% CI: 6% to 19%), (previously 14 %), increases clinical improvement by 14% (95% CI 8% to 18%), reduces progression to invasive ventilation by 15% (95% CI 8% to 22%) (previously 16%), and reduces the time to clinical improvement and to hospital discharge. The expert working group (EWG) noted that these effects were of moderate significance and there is no change in the pooled effect from the previous evidence compilation.
Undesirable effects
The undesirable effects are staying as small, because there is no change in the pooled effects from the previous evidence compilation.
Certainty of evidence
The expert working rated the overall certainty of evidence as moderate.
Values
The reported outcomes of death, clinical improvement at day 28, time to clinical improvement, progression to mechanical ventilation does not have much variability.
Balance of effects
The balance of effects favoured the intervention.
Resources required
The expert working group recognized that there are large direct and indirect costs and resources required to deliver Tocilizumab as an intravenous injection.
Certainty of evidence of required resources
There are no studies evaluating the required resources.
Cost effectiveness
Published evidence was not consulted for this judgement, but the expert working group also acknowledged that this intervention might be variably used in private not-for-profit, private for-profit and public hospitals due to the attendant costs. However, the working group felt the cost-effectiveness probably favoured the use of tocilizumab in a carefully selected sub group of patients with serious or critical COVID-19 disease.
Equity
The working group felt that impact of the use of tocilizumab would probably reduce equity. This is an expensive intervention and hence it is likely to create inequity between those who can afford it versus those who cannot, and in places where it is available freely versus where it is not.
Acceptability
The expert working group felt that, apart from the cost, this is an acceptable intervention as it is an intravenous single dose injection that could reduce mortality without an increase in adverse events.
Feasibility
The working group felt that is feasible to implement this intervention, as tocilizumab has been used widely before in the treatment of chronic rheumatic diseases.
Tocilizumab should only be added on to corticosteroid therapy. The largest trials reported a median CRP >100mg/L (>10mg/dL) and oxygen need. Treatment with tocilizumab was initiated within 24 hours after admission to intensive care in one of the largest trials. Quicker clinical improvement and reduced progression to ventilation with Tocilizumab in these patients may lead to increased hospital bed availability and reduced oxygen use. However, it is an expensive intervention and in short supply, so physicians who prescribe this intervention need to consider the additional benefits over corticosteroids vs. the cost burden it entails.
Tocilizumab should NOT be used in a patient who has an active clinically evident bacterial, mycobacterial, viral or fungal infection as previous experience with use in autoimmune conditions suggests that it may contribute to an increased risk of secondary infections (21). However in the meta-analysis of RCTs studied we did not encounter an increased risk of secondary infections. There are numerous reports of invasive fungal infections and tuberculosis in patients with COVID-19, which are more common when corticosteroids are used, and their risk might be increased further by tocilizumab use. This may be of greater concern for patients with diabetes mellitus (especially with poor glycaemic control), who are immunosuppressed (including due to high-dose corticosteroids) and those who are neutropenic. Thus, we recommend taking into consideration very carefully the type of host and the entire clinical setting to encourage responsible use, and these groups may need closer monitoring.
After administration, it may be prudent to evaluate for infusion reactions, hepatitis, thrombocytopenia, neutropenia and hyperglycaemia and onset of secondary infections.
The expert working group recommends tocilizumab in a specific subgroup of patients with rapidly progressive clinical worsening of severe or critical COVID-19 with evidence of significant systemic inflammation and high oxygen requirement. Disaggregated data for the effects of tocilizumab from the trials on pre-specified subgroups, particularly children, pregnant women and those with chronic kidney or liver disease were not available.
Based on product literature and use for other conditions, tocilizumab should be used with more careful monitoring in patients at high risk of gastrointestinal perforation, and those with hepatic impairment, demyelinating disorders, thrombocytopenia, or neutropenia. Pregnancy data from animals shows an increased rate of congenital malformations and possibly pre-term birth or spontaneous abortions. However, it has been used successfully in pregnancy during this pandemic and so a risk/benefit discussion should take place between clinician and patient. Tocilizumab has also been used widely in children for other conditions, so a risk/benefit discussion should take place regarding its use for children.
Use of tocilizumab and real-world safety should be monitored using audits and registries. Its use in the subgroups mentioned above, and incidence of adverse effects, should be prioritised.
- Though it has been shown that Tocilizumab may improve clinical outcomes when added on to corticosteroids, applicability will only be evident after a cost-benefit analysis in resource limited settings in India. This is an urgent research priority considering the country faced a manpower, oxygen, and intensive care unit bed shortage during the second wave of the covid-19 pandemic in India.
- In addition, the utility of biomarkers such as CRP predicting response in an Indian setting should be studied.
- Further randomized controlled trials should be conducted to enable further precision on the optimal timing for use of Tocilizumab in the course of the COVID-19 illness, and the patient subgroup with maximum benefit and least harm.
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021 Aug 10;326(6):499–518.
- WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2022 Sep 5]. Available from: https://covid19.who.int
- Coomes EA, Haghbayan H. Interleukin‐6 in Covid‐19: A systematic review and meta‐analysis. Rev Med Virol. 2020 Nov;30(6):e2141.
- Tocilizumab: Drug information - UpToDate [Internet]. [cited 2022 Sep 5]. Available from: https://www.uptodate.com/contents/tocilizumab-drug-information?search=tocilizumab&source=panel_search_result&selectedTitle=1~147&usage_type=panel&kp_tab=drug_general&display_rank=1
- Declercq J, Van Damme KFA, De Leeuw E, Maes B, Bosteels C, Tavernier SJ, et al. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet Respir Med. 2021 Dec 1;9(12):1427–38.
- Broman N, Feuth T, Vuorinen T, Valtonen M, Hohenthal U, Löyttyniemi E, et al. Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers; COVIDSTORM—a prospective, randomized, single-centre, open-label study. Clin Microbiol Infect. 2022 Jun 1;28(6):844–51.
- Remap-Cap investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021 Apr 22;384(16):1491–502.
- Hermine O, Mariette X, Porcher R, Resche-Rigon M, Tharaux PL, Ravaud P. Effect of Interleukin-6 Receptor Antagonists in Critically Ill Adult Patients with COVID-19 Pneumonia: two Randomised Controlled Trials of the CORIMUNO-19 Collaborative Group. Eur Respir J. 2022 Jan 1;2102523.
- Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Madjlessi Simon T, Porcher R, et al. Tocilizumab Plus Dexamethasone in Patients with Moderate-to-Severe COVID-19 Pneumonia: a Randomized Clinical Trial of the CORIMUNO-19 Study Group [Internet]. Rochester, NY; 2021 [cited 2022 Jun 28]. Available from: https://papers.ssrn.com/abstract=3909736
- Abani O, Abbas A, Abbas F, Abbas M, Abbasi S, Abbass H, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2021 May;397(10285):1637–45.
- Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med. 2021 Apr 22;384(16):1503–16.
- Rosas IO, Diaz G, Gottlieb RL, Lobo SM, Robinson P, Hunter BD, et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med. 2021 Nov 1;47(11):1258–70.
- Rutgers A, Westerweel PE, van der Holt B, Postma S, van Vonderen MGA, Piersma DP, et al. Timely Administration of Tocilizumab Improves Survival of Hospitalized COVID-19 Patients [Internet]. Rochester, NY; 2021 [cited 2022 Jun 28]. Available from: https://papers.ssrn.com/abstract=3834311
- Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021 Jan 7;384(1):20–30.
- Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021 Jan 1;181(1):24–31.
- Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021 May;9(5):511–21.
- Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020 Dec 10;383(24):2333–44.
- Talaschian M, Akhtari M, Mahmoudi M, Mostafaei S, Jafary M, Husseini AS, et al. Tocilizumab Failed to Reduce Mortality in Severe COVID-19 Patients: Results From a Randomized Controlled Clinical Trial. In 2021.
- Veiga VC, Prats JAGG, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021 Jan 20;372:n84.
- Wang D, Fu B, Peng Z, Yang D, Han M, Li M, et al. Tocilizumab Ameliorates the Hypoxia in COVID-19 Moderate Patients with Bilateral Pulmonary Lesions: A Randomized, Controlled, Open-Label, Multicenter Trial [Internet]. Rochester, NY; 2020 [cited 2022 Jun 28]. Available from: https://papers.ssrn.com/abstract=3667681
- Morel J, Constantin A, Baron G, Dernis E, Flipo RM, Rist S, et al. Risk factors of serious infections in patients with rheumatoid arthritis treated with tocilizumab in the French Registry REGATE. Rheumatology. 2017 Oct 1;56(10):1746–54.
Covid Management Guidelines India Group – Anti-inflammatory and Antibody working Group - Tocilizumab. Covid Guidelines India; Published online on October 04th, 2022; URL: https://indiacovidguidelines.org/tocilizumab-2/
