This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION:
We do not recommend Casirivimab and Imdevimab (REGEN-COV™️) for people with mild COVID-19 infection and no risk factors for progression to severe disease due to the involved expense and low likelihood of progression to severe disease. However, Casirivimab and Imdevimab (REGEN- COV™️) may be given in people with mild COVID-19 infection with one or more risk factors (see list below) to prevent progression to severe disease within the first ten days of the illness, irrespective of antibody status.
DATE OF RECOMMENDATION: 10/08/2021
Definition of mild illness:
Symptomatic (any acute COVID-19 related symptoms)
AND respiratory rate <24/min
WITHOUT pneumonia or hypoxia
A conditional recommendation is one for which the panel is less confident about the balance between desirable and undesirable effects of the intervention, or other aspects, such as cost and feasibility.
Definition of mild illness:
Symptomatic (any acute COVID-19 related symptoms)
AND respiratory rate <24/min
WITHOUT pneumonia or hypoxia
Risk factors
1. Age >50 years
2. Obesity, defined as body mass index >30
3. Cardiovascular disease, including hypertension
4. Chronic lung disease, including asthma
5. Chronic metabolic disease, including diabetes
6. Chronic kidney disease, including those on dialysis
Casirivimab/Imdevimab (REGEN-COV™) antibody cocktail is an investigational product developed by Regeneron pharmaceuticals as a therapeutic agent. Therapeutics that remain effective against emerging SARS-CoV-2 variants of concern (VOCs), which evade immunity from prior SARS-CoV-2 infection, vaccination, and some monoclonal antibodies (3,4,5) are the need of the hour. REGN-COV™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor.
In mild COVID patients with > 1 risk factor and without hypoxia, REGEN-COV™ antibody cocktail at a dose of 600/600mg was evaluated within 10 days of symptom onset. Moderate quality evidence revealed that REGEN-COV™ in doses of 1.2g,2.4g and 8.0g reduced hospital admission. In addition, low quality of evidence revealed that it may reduce length of stay. However, mortality was not reduced with any dose of REGEN-COV™ though the evidence was of very low quality. It did not also reduce progression to invasive mechanical ventilation with doses of 1.2g and 2.4g but the evidence was again of very low quality. It was also noted that REGEN-COV™ did not cause any major adverse effects suggesting that it is safe. However, it is an expensive drug and needs to be prudently used to maximize cost effectiveness. Hence this drug needs to be used cautiously in a population that is likely to benefit maximally i.e., those who are high risk of disease progression
Date of latest search: 22nd June 2021
Date of completion & presentation to Expert Working Group: 29th June 2021
Date of planned review: 29th December 2021
Evidence synthesis team: Hanna Alexander, Naveena George, Richard Kirubakaran, Bhagteshwar Singh and Priscilla Rupali
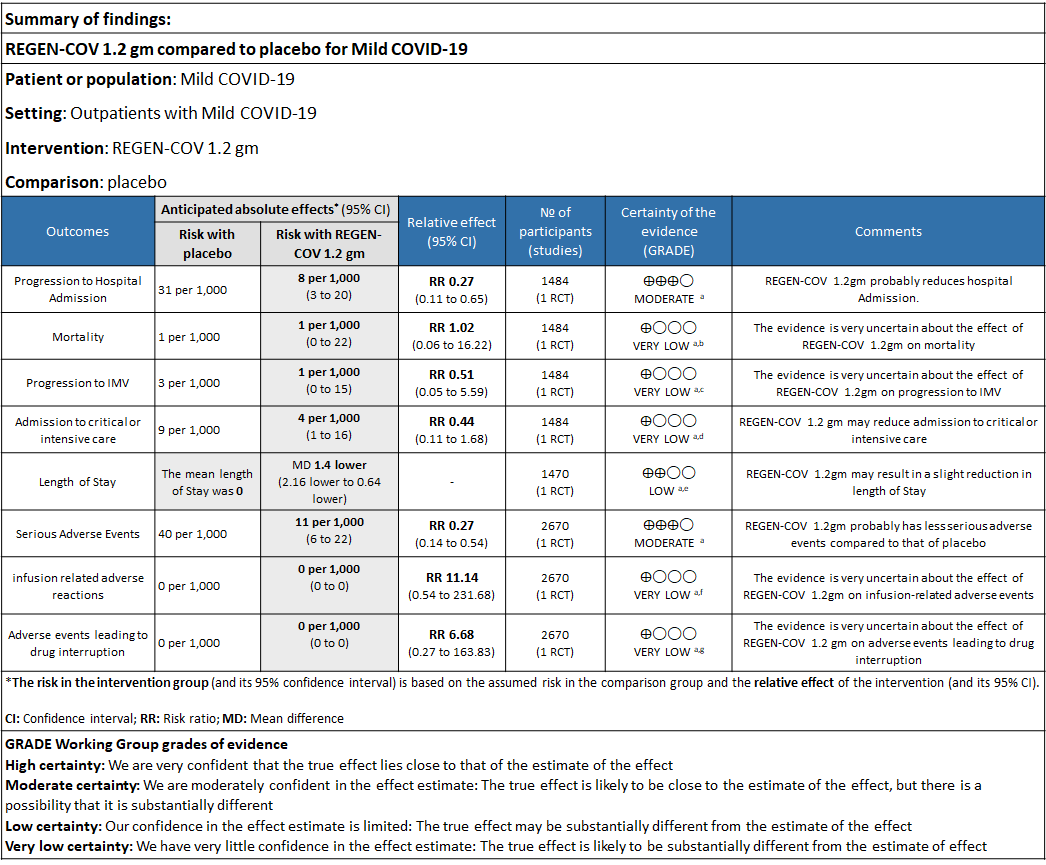
Explanations
a. Downgraded for one level for serious risk of bias as the study reported some concerns
b. Downgraded by two levels for serious imprecision.95% CI ranges from 0.06 to 16.22 and the event rate is very low.(OIS inadequate)
c. Downgraded by two levels for serious imprecision.95% CI ranges from 0.05 to 5.59 and the event rate is very low.(OIS inadequate)
d. Downgraded by two levels for serious imprecision.95% CI ranges from 0.11 to 1.68 and the event rate is very low.(OIS inadequate)
e. Downgraded by one level for serious imprecision 95% CI ranged from 0.64 to 2.16 which is not clinically interpretable.
f. Downgraded by two levels for serious imprecision.95% CI ranges from 0.54 to 231.68 and the event rate is very low.(OIS inadequate)
g. Downgraded by two levels for serious imprecision.95% CI ranges from 0.27 to 163.83 and the event rate is very low.(OIS inadequate
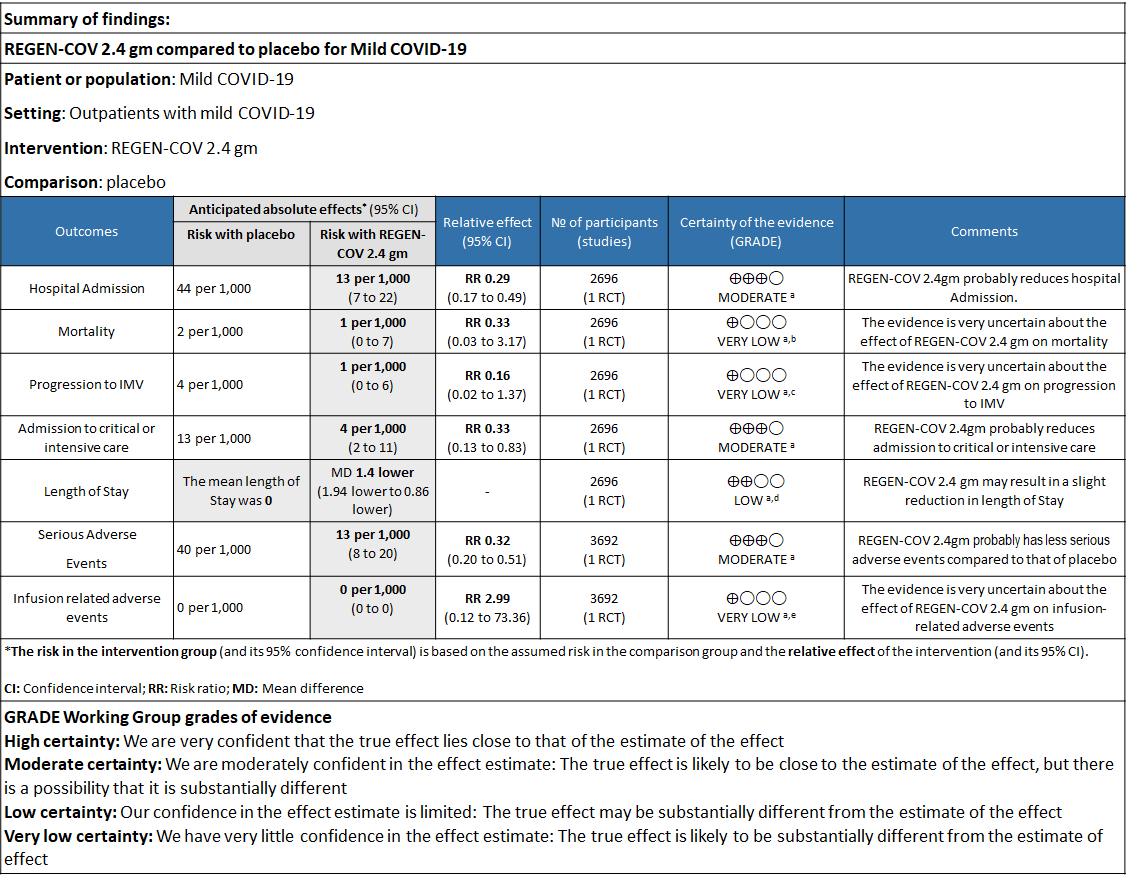
Explanations
a. Downgraded for one level for serious risk of bias as the study reported some concerns
b. Downgraded by two levels for serious imprecision.95% CI ranges from 0.03 to 3.17 and the event rate is very low.(OIS inadequate)
c. Downgraded by two levels for serious imprecision.95% CI ranges from 0.02 to 1.37 and the event rate is very low.(OIS inadequate)
d. Downgraded by one level for serious imprecision as the decrease was 1.4 days lower but 95% CI ranged from 0.86 to 1.94 which is not clinically relevant
e. Downgraded by two levels for serious imprecision.95% CI ranges from 0.12 to 73.36 and the event rate is very low.(OIS inadequate)
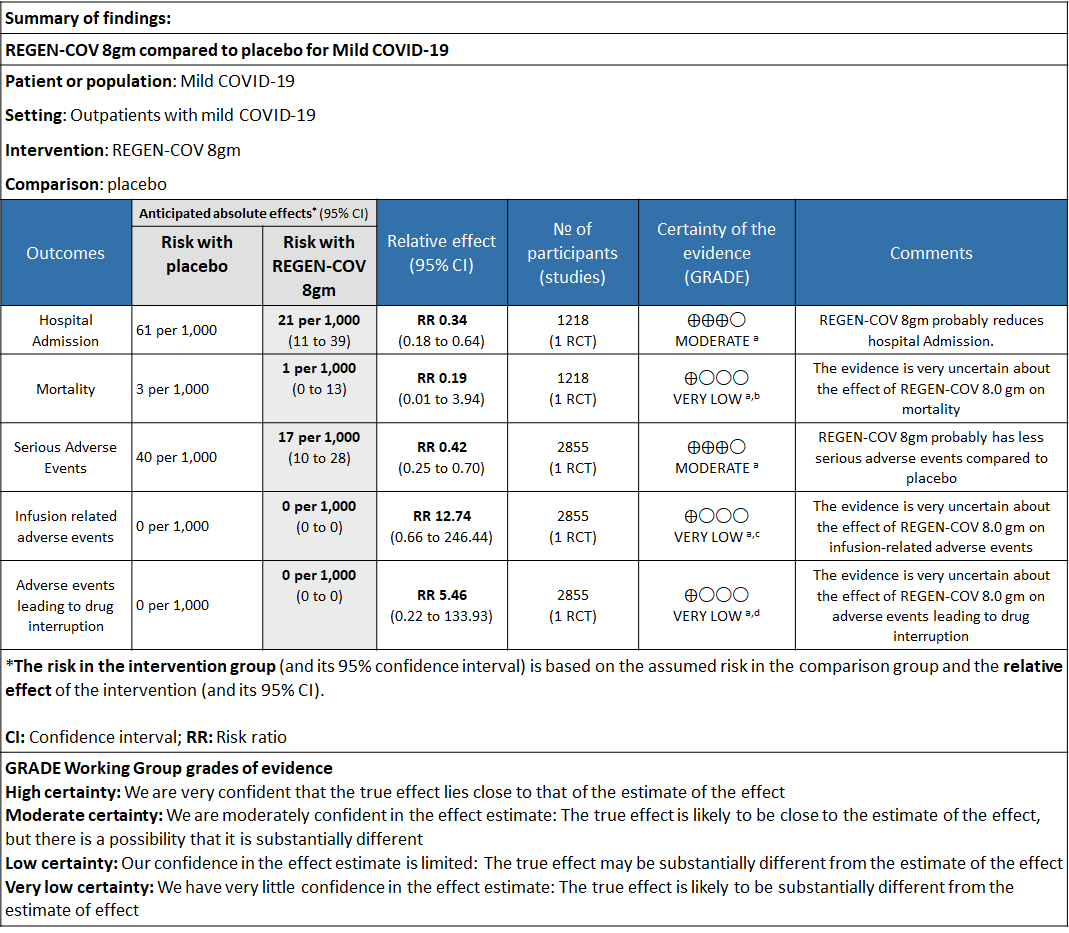
Explanations
a. Downgraded for one level for serious risk of bias as the study reported some concerns
b. Downgraded by two levels for serious imprecision.95% CI ranges from 0.01 to 3.94 and the event rate is very low.(OIS inadequate)
c. Downgraded by two levels for serious imprecision.95% CI ranges from 0.66 to 246.44 and the event rate is very low.(OIS inadequate)
d. Downgraded by two levels for serious imprecision.95% CI ranges from 0.22 to 133.93 and the event rate is very low.(OIS inadequate)
REGEN-COV™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor(1). The combination is manufactured by Regeneron pharmaceuticals. The drug was approved by the FDA for emergency use on 21 November 2020 in patients ≥12 yrs. of age, weighing at least 40 kilograms with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19. The authorized dosage is 1,200 mg of Casirivimab and 1,200 mg of Imdevimab administered together as a single intravenous (IV) infusion over 60min as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset. (Optimal dosing regimen is not established yet)(2).The CDSCO in India, on 5 May 2021, granted an Emergency use authorization (EUA) to Roche (Genentech) and Regeneron for use of REGEN-C0V™ in the country. The announcement came in light of the second wave of the COVID-19 pandemic in India(3). This review aims to provide a summary of the available evidence from randomized clinical trials of REGEN-COV™ for treatment of COVID-19 which could guide clinicians and researchers regarding the appropriate use of this drug in the future
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 28 June 2021.
We searched the above databases and found 11 records. After removing duplicates and excluding studies that did not match our PICO question, only one randomized controlled trial was found and included for the analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Admission into hospital (all cause)
- Time to clinical recovery
- Progression to severe disease- requiring O2, mechanical ventilation or ICU care
- Important (secondary):
- Time to viral clearance (negative PCR)
- Death
- Adverse events:
- All
- Serious
- Infusion-related adverse events (if reported, record rate of infusion used)
Two reviewers independently evaluated and validated search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, by one reviewer and checked by a second reviewer and compared with covid NMA database. If there was a difference in more than one domain it was assessed by a third independent reviewer and a consensus obtained.We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs) using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
We found one RCT that matched the PICO question as pre-defined by the expert working group. The trial included a total of 4057 participants, all of whom were adult out patients. This study was done in 82 centers in United States and Mexico. The results are described below; characteristics of the trials are summarized in the Summary of characteristics of included trials table.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Casirivimab and Imdevimab combination (REGEN-COV™) vs Placebo in out patients with mild COVID illness with >1 risk factor for disease progression.
Outcomes
As presented in the ‘Summary of findings’ tables, the evidence ranged from low to very low certainty for the effect of Casirivimab and Imdevimab combination (REGEN-COV) 1.2 gm on admission to critical care, length of stay, mortality, progression to Invasive mechanical ventilation, infusion related adverse events and adverse reactions leading to drug interruption. Moderate certainty of evidence for hospital admission and serious adverse events.
Since this was an adaptive phase 3 randomized controlled trial it compared patients in the doses of 1.2g, 2.4g and 8.00g
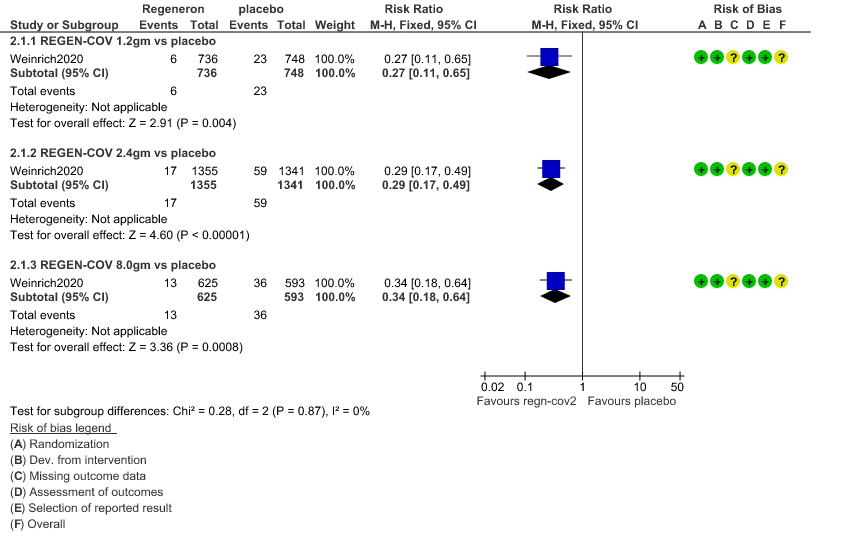
a. Admission to Hospital: Moderate certainty of evidence in 1484 patients belonging to 1.2gm arm (RR 0.27; 95% CI 0.11 to 0.65), 2696 patients in 2.4gm arm (RR 0.29; 95% CI 0.17 to 0.49) and 1218 patients in 8gm arm (RR 0.34; 95% CI 0.18 to 0.64) of Casirivimab-Imdevimabin the selected trial revealed that it reduced admission to hospital in out patients with mild illness (1) .The number needed to treat to prevent one hospital admission included 44, 36 and 26 with doses of 1.2g,2.4g and 8.0g respectively.
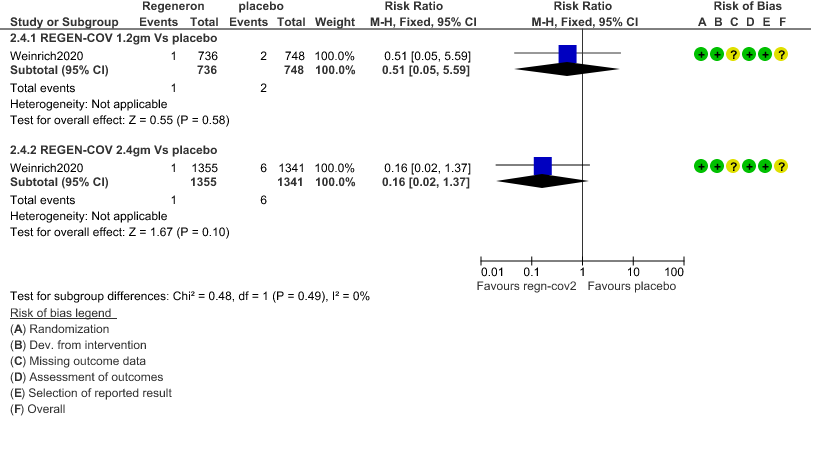
b. Progression to Invasive mechanical ventilation: Very low certainty of evidence in 1484 patients 1.2gm arm (RR 0.51; 95% CI 0.05 to 5.59), 2696 patients in 2.4gm arm (RR 0.16; 95% CI 0.02 to 1.37) and revealed that there was a very uncertain impact on progression to invasive mechanical ventilation.
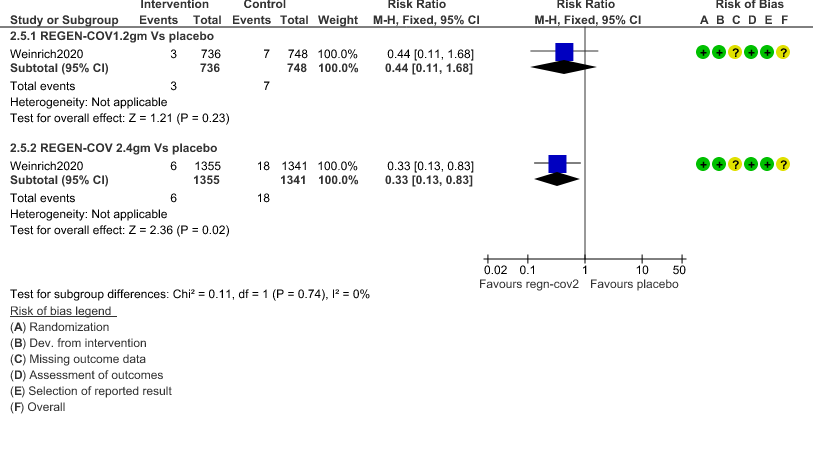
c. Admission to critical or intensive care for any reason:Low certainty of evidence in 1484 patients (RR 0.44; 95% CI 0.11 to 1.68) revealed that 1.2g of REGN-COV did not prevent admission to critical or intensive care.
However, moderate certainty of evidence in 2696 patients in 2.4gm arm (RR 0.33; 95% CI 0.13 to 0.83) showed that REGN-COV at this doseprobably reduces admission to critical or Intensive care.
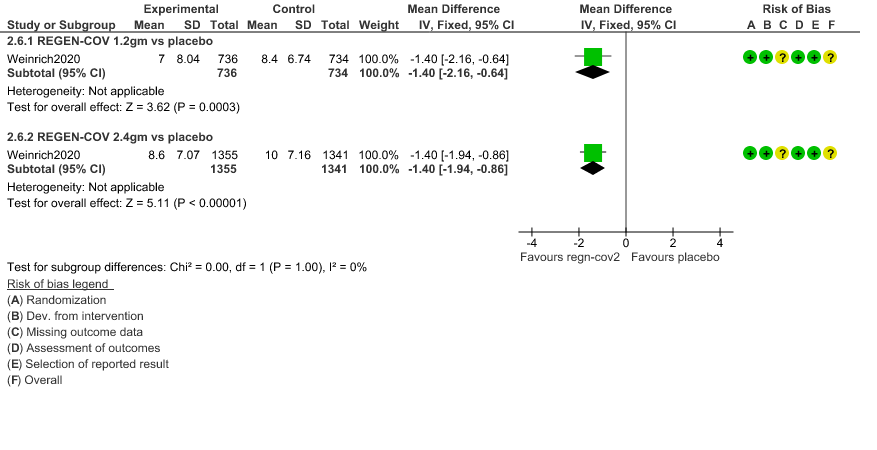
d. Duration of hospitalization: Low certainty of evidence in 1,470 patients in the 1.2g dose and 2696 patients in the 2.4 gm dose of REGEN-COV may reduce duration of hospitalization by a mean difference of 1.4 days when compared to placebo. The median difference in the 1.2g dose arm was 1.5 days and in the 2.4g dose arm was 1 day.
e. All-cause mortality: Very low certainty of evidence in 1484 patients in 1.2gm arm (RR 1.02; 95% CI 0.06 to 16.22), 2696 patients in 2.4gm arm (RR 0.33; 95% CI 0.03 to 3.17), 1218 patients in 8gm arm (RR 0.19; 95% CI 0.01 to 3.94), found that REGEN-COV in doses of 1.2gm and 2.4gm, 8gm has a very uncertain effect on reducing mortality.
f. Adverse events:
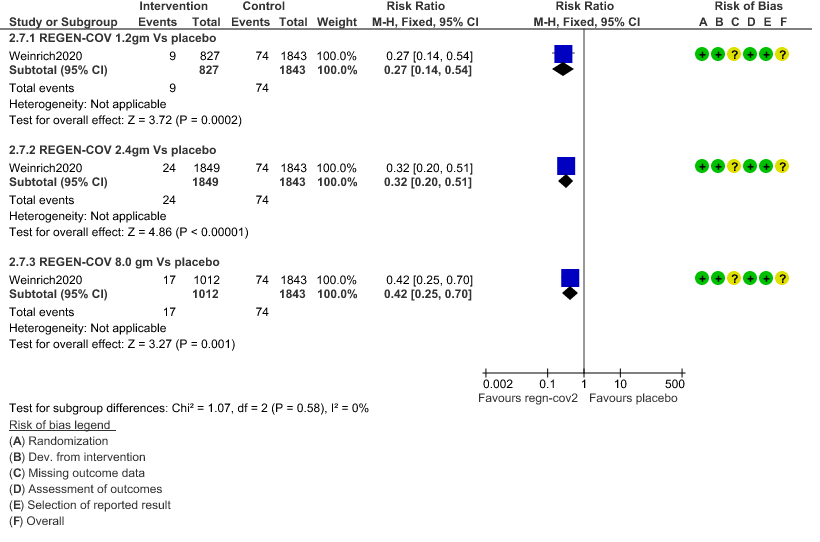
1. Serious Adverse events: Moderate certainty of evidence in 2670 patients in the 1.2gm arm (RR 0.27; 95% CI 0.14 to 0.54), 3692 patients in 2.4gm arm (RR 0.32; 95% CI 0.2 to 0.51) and 2855 patients in 8gm arm (RR 0.42; 95% CI 0.25 to 0.7) found that REGEN-COV in doses of 1.2gm, 2.4gm and 8 gm probably has less serious adverse events compared to placebo.
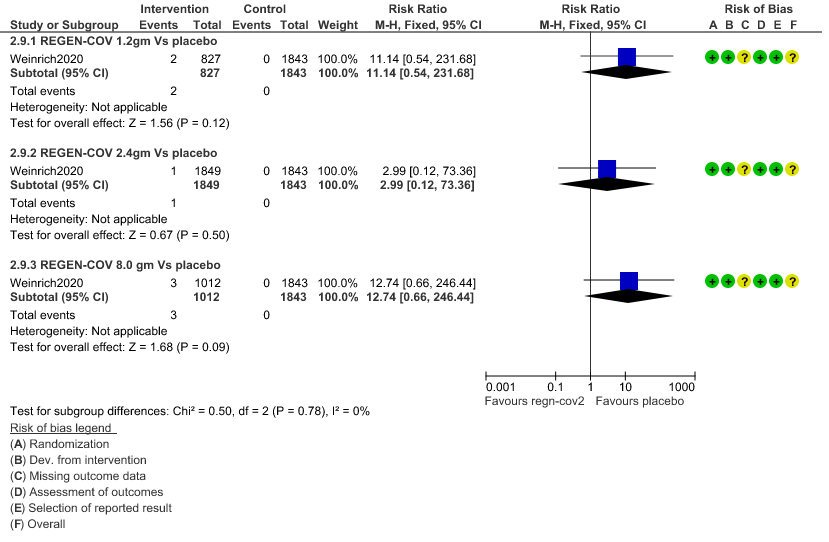
2. Infusion related adverse events: Very low certainty of evidence in 2670 patients 1.2gm arm (RR 11.14; 95% CI 0.54 to 231.68), 3692 patients in 2.4gm arm (RR 2.99; 95% CI 0.12 to 73.36) and 2855 patients in 8gm arm (RR 12.74; 95% CI 0.66 to 246.44) revealed that evidence was very uncertain that REGEN-COV 1.2gm, 2.4gm and 8 gm has less infusion related adverse events compared to placebo.
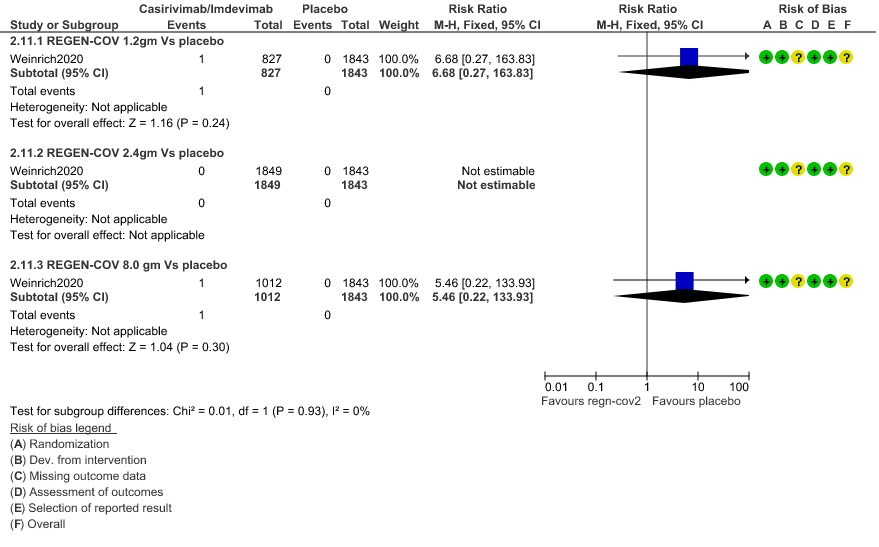
3. Adverse events leading to drug interruption: Very low certainty of evidence in 2670 patients 1.2gm arm (RR 6.68; 95% CI 0.27 to 163.83), and 2855 patients in 8gm arm (RR 5.46; 95% CI 0.22 to 133.93) revealed a very uncertain effect that REGEN-COV 1.2gm and 8 gm had less adverse events that lead to drug interruption as compared to placebo.
g. Time to clinical recovery: The median time to resolution of symptoms in both REGEN-COV dose arms (1.2g and 2.4g) vs placebo was 10 vs 14 days (p<0.0001). - If the viral load was > 6 log copies then Regeneron did reduce symptoms faster by 5 days (p<0.0001)
- If viral load was < 6 log copies it may reduce symptoms but by only 2 days. (p= not significant)
- If Ab negative at baseline, either dose reduced symptoms by 3 to 4 days. (p=<0.0002)
- If Ab positive at baseline 2.4g dose of Regeneron may reduce symptoms by 4 days (p=0.05)
h. Difference in Viral load:Time to negative PCR as specified by the PICO was not available. However, a difference in viral loads was measured. In the modified full analysis set (mFAS) the viral load was 0.75 log less with either 1.2g or 2.4g dose of REGN-COV which is not clinically relevant.
- In those with a baseline viral load >6 log copies, there was a 1 log drop in the viral load when 1.2g (1.01 log copies) or 2.4g (1.04 log copies) of REGN-COV was used vs placebo
- If seronegative at baseline, there was a drop in the intervention arm by 1 log copies, when 1.2g (0.86 log copies) or 2.4g (1.04 copies) dose of REGN-COV was used.
- In those seropositive at baseline with 1.2g (0.16 log copies) or 2.4g (0.43 log copies) the drop in viral load was clinically and statistically insignificant.
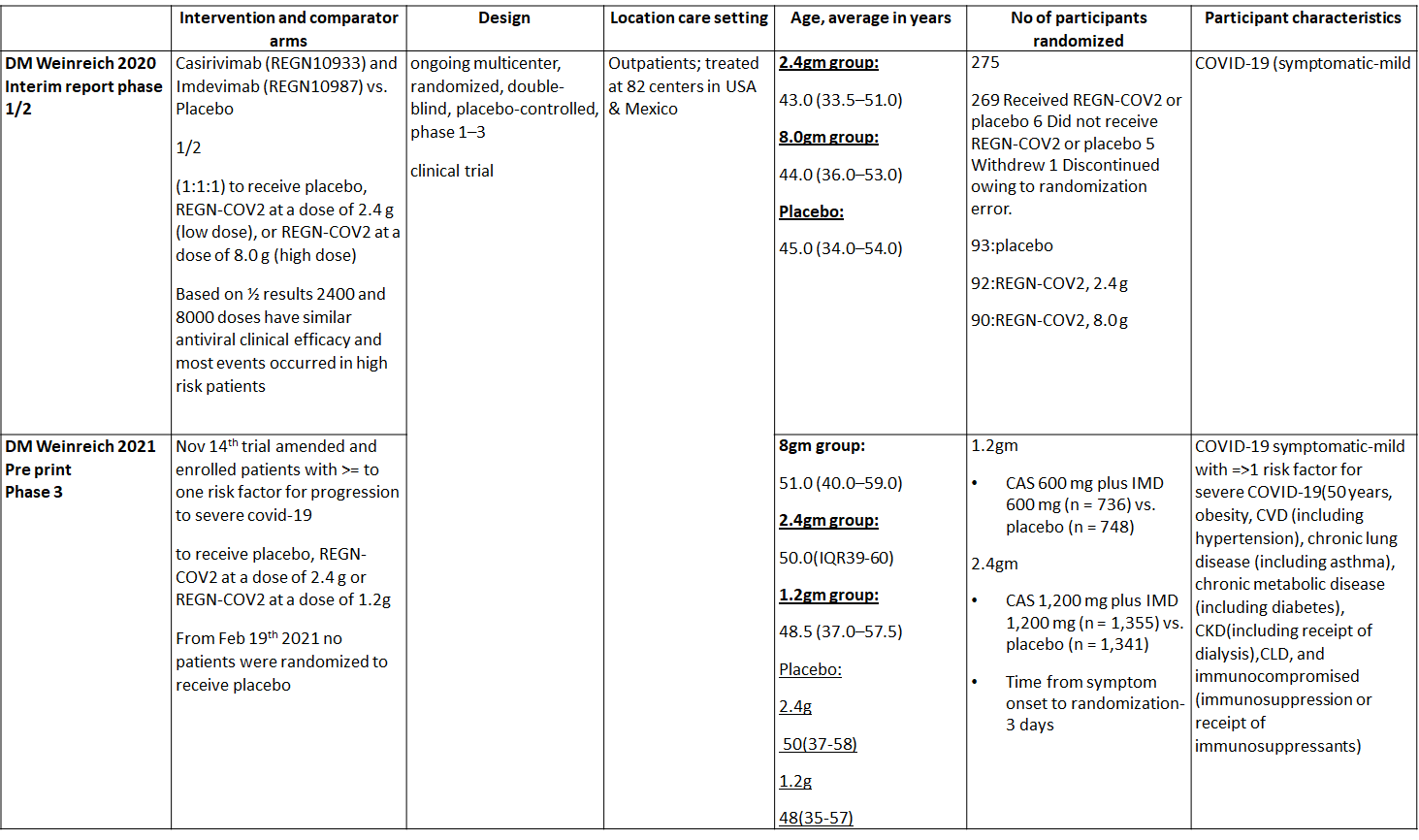
1. Progression to Hospital admission (each dose)
2. Progression to Invasive mechanical ventilation
3. Admission to critical care/Intensive care unit
4. Duration of Hospital stay (Length of Stay)
5. Mortality
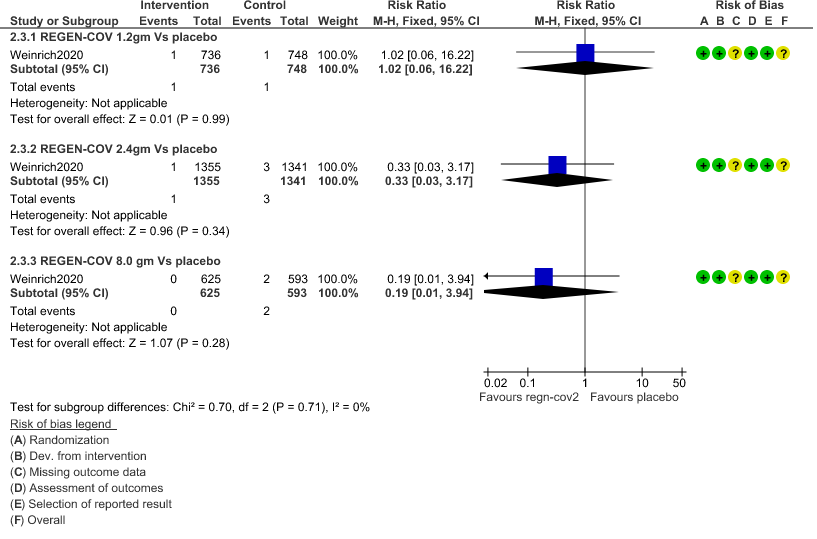
6. Adverse Events
a. Serious Adverse events
b. Infusion related adverse events
c. Adverse events leading to drug interruption
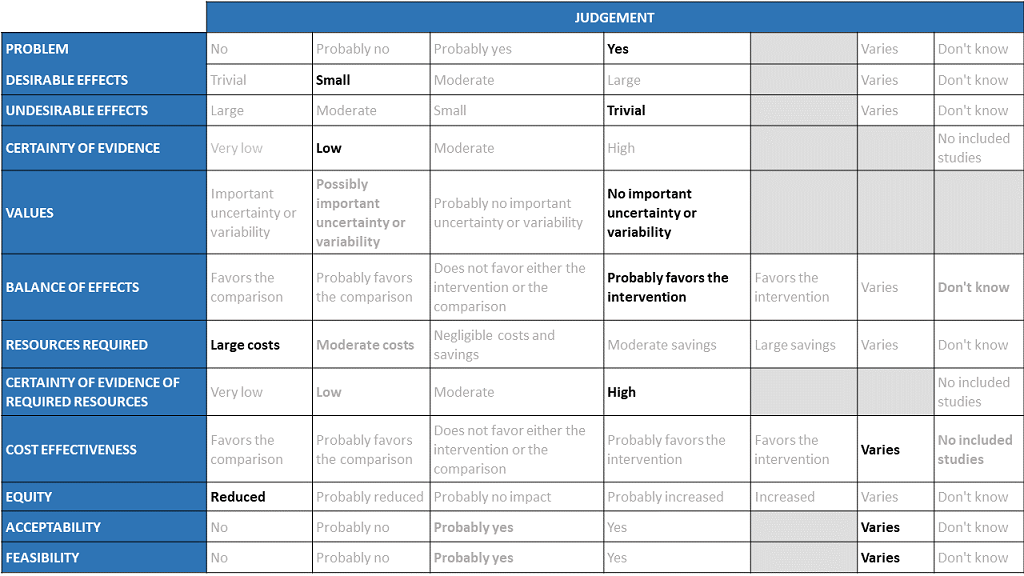
The Antibody Expert Working Group met on 29th June 2021 to consider Casirivimab and Imdevimab combination 1.2g as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Casirivimab and Imdevimab.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India, has resulted in more than 30 million infections and 0.39 million deaths, wreaking havoc on the country's health system. During the second wave believed to be due to the delta variant, the country experienced a significant health crisis due to a lack of intensive care unit beds, oxygen, and skilled manpower. This resulted in many irrational and experimental treatment interventions. Casirivimab and Imdevimab an antibody cocktail was developed by Regeneron pharmaceuticals as an experimental therapy which would be effective in patients across different categories of severity even when the infections were caused by variant or mutant strains. Studies have been done in 3 categories of severity -asymptomatic, mild out-patients and in the severe-critical category. With so many therapeutic options now available it becomes incumbent upon us as a community to define which population would maximally benefit from this drug keeping costs in mind. This expensive drug is now available in India in the dose of 1.2g (costing Rs 60,000) and it would be prudent to synthesize evidence to promote judicial use of the same. The group judged the problem to be of utmost priority.
Desirable effects
The panel discussed the anticipated desirable effects of the drug in covid-19. It was noted that the drug reduced progression to hospital admission in individuals with more than one risk factor and that was considered to be significant as that would definitely reduce requirements for advanced hospital care However, the effect size is low. Length of stay was found to be 1.4 days lower in REGEN-COV™ group vs placebo (1.2 vs placebo) which also the group felt was important in the midst of an acute shortage of beds. The median time to resolution of Covid-19 symptoms was also 4 days shorter vs placebo (10 vs 14 days; p6 log copies or if seronegative at baseline there was a drop in 1 log copies in the intervention arm as compared to placebo. There is no impact on progression to Invasive mechanical ventilation.
Undesirable effects
The panel felt that there was very little evidence to suggest that the undesirable effects are substantial. In the evidence reviewed there were very few adverse events overall and also those requiring interruption of the intervention.
Certainty of evidence
Using GRADE methodology, the group rated the certainty of evidence as moderate for progression to hospital admission and serious adverse events; low certainty for length of stay and very low certainty for mortality, progression to Invasive mechanical ventilation, admission into intensive care unit, infusion related adverse events and events leading to drug interruption. The panel felt that the overall certainty of evidence reviewed was low.
Values
The panel reviewed the outcomes that were studied and concluded that there is no important uncertainty or variability on how much people would value these.
Balance of effects
The panel felt that the balance of effects favors the intervention. The group felt that the trend favored the intervention since there is moderate certainty evidence that the intervention may reduce hospital admissions and length of stay in mild disease with few adverse events. The group felt that this was a significant effect as it could potentially free up hospital beds for those at a high risk of progression.
Resources required
The panel felt that the resources required to implement this intervention are large as the cost also factors the drug cost and the resources required to administer this drug safely as an intravenous infusion in a healthcare setting while maintaining all the infection control precautions.
Certainty of evidence of required resources
The panel felt that no studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
Published evidence was not consulted for this judgment. The work group acknowledged that implementation of this intervention might vary among patients, hospital administrators and clinicians in different economic classes of patients due to the considerably large cost of the drug itself and the resources required.
Equity
The working group felt that the use of the intervention would definitely reduce equity. This is an expensive intervention and hence it is likely to create inequity between those who can afford it versus those who cannot, and in places where it is available freely versus where it is not.
Acceptability
The panel felt that the acceptability of the intervention among different stakeholders would vary because of the clinical efficacy stems from one trial which might lead to some reluctance but also the involved costs.
Feasibility
The panel felt that the feasibility of implementing this intervention varies depending on the stakeholder and all the factors discussed above should be carefully considered.
Casirivimab Imdevimab is an antibody cocktail which has shown to reduce hospital admission when used in mild COVID-19 infection in those patients with more than 1 risk factor for disease progression. This has been found in a single randomized trial done in the USA. There are other trials underway in this subgroup of patients. This intervention needs to be delivered intravenously over one hour and hence likely will require a health care system/clinic for drug administration with appropriate infection control protocols in place. In addition, this drug is quite expensive even if the lowest dose of efficacy is chosen which would be 1.2g; the cost and system charges are likely to be out of reach of the common man. Hence judicious use of this drug in the appropriate clinical setting is crucial. At this point, only the intravenous route is advised in patients with mild COVID-19 illness and since 2.4g is almost equivalent in efficacy to the 1.2g in most places the 1.2g dose is recommended commonly. There has been some concern that with mutating variant strains that this drug may have reduced efficacy, however evidence is slowly emerging that this antibody cocktail maintains its efficacy even in the presence of the current circulating variant strains (6). Various pharmaceutical companies have obtained permission for this drug to be licensed and marketed in India as well (7).
Being a protein moiety, though the reported drug related reactions were very low, anaphylaxis and other allergic reactions have been reported. The costs of implementing this drug are considerable and therefore it’s widespread use in the Indian setting will not be practical or feasible. However, the use should be definitely be considered in select sub-groups, where there are no cost constraints or in clinical settings where it would be considered cost effective. Hence, in the mildly symptomatic group with significant comorbidities or > 1 risk factor, the drug could be considered in those who can afford it if used within 7 days of symptom onset.
This drug is used only in those with mild illness in a dose of 1.2g intravenously to prevent hospital admissions and reduce length of stay. Though higher doses of 2.4g and 8.0g were also evaluated they offered no additional benefit to a dose of 1.2g in this severity subgroup. The 2.4g dose in addition also reduced admission or progression to critical or intensive care.
Though antibody status was evaluated in this study, the benefits of reduction in hospital admission and decrease in length of stay seemed to be irrespective of antibody status. The viral load reduction and time to clinical recovery seemed to be faster in those found to be seronegative as compared to the seropositive individuals.
Viral load difference in those with a baseline of > 6 log copies and < 6 log copies were also evaluated and symptom resolution was significantly quicker in those given the intervention with the effect being pronounced in the former.
This conditional recommendation for use of 1.2 gm of the casirivimab imdevimab cocktail may be revisited with emerging evidence. The group will continue to monitor new data about efficacy of the cocktail against variants including the delta and delta plus variant and whether an increase in dose of the cocktail is warranted. The emergence of data about efficacy of the cocktail in patients with multiple risk factors or a combination of risk factors with clinical symptoms with results of lab investigations may allow more cost-effective use of the drug. With increasing availability of the drug in India, cohort studies about clinical outcomes and drug adverse effects are likely to be published which the group will need to evaluate to arrive at recommendations which are contextualized to the Indian setting with the attendant cost constraints.
Several interventions like steroids and IL-6 inhibitors have been shown to decrease mortality in moderate, severe and critical categories of COVID-19 infections. However, most of these interventions have been studied as an add on to steroids and have not been evaluated separately.
1. Studies to determine if Casirivimab Imdevimab combination is cost effective in mild COVID-19 infection.
2. Defining the optimal high risk group where this drug could be given to obtain maximal therapeutic benefit.
3. Use of this drug in the various risk groups to evaluate the efficacy of this drug
4. Continued surveillance to ensure sustained efficacy of this drug in the presence of new evolving mutational variants.
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med [Internet]. 2020 Dec 17 [cited 2021 Jun 3]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa2035002
- Commissioner O of the. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 [Internet]. FDA. FDA; 2020 [cited 2021 Jun 29]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19.
- Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 2021;372:n579.
- Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021.
- Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med 2021.
- Regeneron EUA HCP Fact Sheet 06032021. FACT SHEET FOR HEALTH CARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF REGEN-COVTM (casirivimab and imdevimab) accessed from https://www.fda.gov/media/145611/download
- Roche receives Emergency Use Authorisation in India for its investigational Antibody Cocktail (Casirivimab and Imdevimab) used in the treatment of Covid-19 | Cipla [Internet]. [cited 2021 Jun 29]. Available from: https://www.cipla.com/press-releases-statements/roche-receives-EUA-India-investigational-antibody-cocktail-casirivimab-Imdevimab-covid.
Covid Management Guidelines India Group - Anti-body Working Group. Casirivimab – Imdevimab. Covid Guidelines India; Published online on August 11, 2021; URL : https://indiacovidguidelines.org/casirivimab-and-imdevimab-regn-covtm/ (accessed ).
