Be this recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: In people with mild to moderate Covid-19 infection and no risk factors, we recommend against using Molnupiravir (strong recommendation). Molnupiravir may be considered as an option only for patients with mild to moderate Covid-19 infection at home who are unvaccinated and have risk factors for severe disease, especially obesity (conditional recommendation).
DATE OF RECOMMENDATION: 16th February 2022
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
A conditional recommendation is one for which the desirable effects probably outweigh the undesirable effects (weak recommendation FOR an intervention) or undesirable effects probably outweigh the desirable effects (weak recommendation AGAINST an intervention) but appreciable uncertainty exists. This implies that not all will be best served by this recommendation and decisions can be made by the patient and caregiver based on patient values, resources and setting with a clear understanding of the ensuing harms and benefits.
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
Molnupiravir is the first oral antiviral developed for treatment of COVID-19 by Merck, Sharp and Dohme and Ridgeback therapeutics.1In November 2021 it was granted conditional authorization by Britain for use in mild to moderate COVID-19 infection2. In December 2021, FDA granted emergency use authorization for treatment of mild COVID-19 infection.3
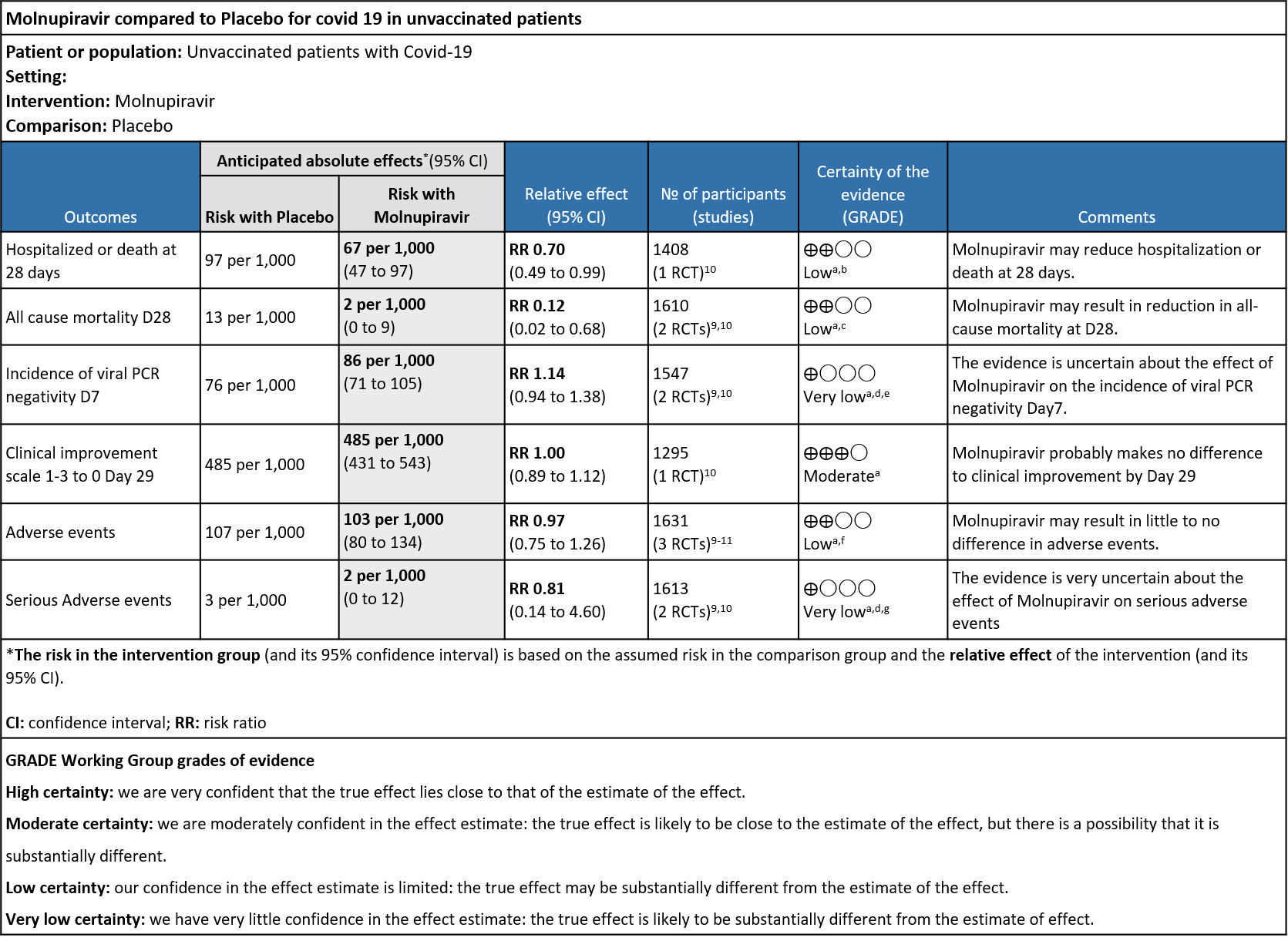
Low certainty evidence from available randomized controlled trial data suggests that Molnupiravir may reduce hospitalization or death in unvaccinated individuals. A subgroup analysis suggested that this beneficial effect was mainly seen in obese patients.7 Given that the globally circulating COVID-19 variant now is Omicron which predominantly produces mild disease resulting in very few instances of hospitalization or death,4the utility of this antiviral in the present scenario remains suspect. The group decided on a conditional recommendation against using Molnupiravir as trial data suggested a smaller benefit of Molnupiravir when compared with other agents (like Paxlovid or Remdesivir) in unvaccinated individuals infected with an older variant of COVID-19. The NNT of 35 was considered very large and therefore treatment does not appear to be justified considering possible long-term repercussions on teratogenicity, gastrointestinal adverse effects, cost & unknown effects on viral mutagenesis. There may be a small subset of obese, unvaccinated, immunocompromised (even if vaccinated) patients having multiple risk factors or aged>60 years unwilling for IV Remdesivir who may benefit from addition of Molnupiravir. In addition, there may be added uncertainties with regard to vaccine type & efficacy, number of doses taken, duration since last dose of vaccination as well as mitigating factors like herd, convalescent or vaccine induced immunity or changed properties of the virus which may also influence use of Molnupiravir in COVID-19 infection.
Date of latest search: 02nd January 2022
Date of completion & presentation to Expert Working Group: 10th January 2022
Date of planned review: 10th July 2022
Evidence synthesis team: Hanna Alexander, Jane Miracline, Richard Kirubakaran, Priscilla Rupali
Explanations
a. Downgraded by one level for serious indirectness as the study population is unvaccinated which does not reflect the current scenario
b. Downgraded by one level for serious imprecision as the lower bound of the 95% CI includes no difference and the number of events and participants do not satisfy the requirements of the optimal information size.
c. Downgraded by one level for serious imprecision as the 95% CI ranges from 0.02-.068. Sample size did not meet the OIS criteria
d. Downgraded by one level for high risk of bias in selected studies (Fischer)
e. Downgraded by one level for serious imprecision as the 95% CI crosses 1(0.94-1.38)
f. Downgraded by one level for serious imprecision as the 95% CI crosses 1(0.75-1.26)
g. Downgraded by one level for serious imprecision as the 95% CI is wide (0.14-4.60)
Molnupiravir is a newer orally bioavailable prodrug of Emory Institute of Drug Development-2801 [EIDD-2801]/MK-4482), which is the synthetic ribonucleoside derivative N4-hydroxycytidine and ribonucleoside analog, with potential antiviral activity against a variety of RNA viruses that has been recently been tested in COVID-19. The TP form of EIDD-1931 is incorporated into RNA and inhibits the action of viral RNA-dependent RNA polymerase. This results in the termination of RNA transcription decreases viral RNA production, and viral RNA replication1. The drug was invented at Emory university for viral diseases of concern and was later was acquired by Ridgeback therapeutics in partnership with Merck & Co, USA. Molnupiravir was initially developed as a possible treatment for influenza viruses, encephalitic alphaviruses like Venezuelan, Eastern and Western equine encephalitic viruses due to its significant inhibitory effect in cell cultures5. On December 23rd 2021 FDA gave emergency use authorization for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults (>18yrs) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate. The drug is indicated to be initiated within 5 days of symptom onset6.On December 2021 DCGI gave emergency use authorization for the antiviral in India for the treatment of adult patients with Covid-19 who are at a high risk of progression to severe disease.Molnupiravir’s mechanism against Covid-19 has some experts concerned about its mutagenic potential in human cells. An in vitro study by Zhou et al has suggested that the drug, though intended to disrupt only viral RNA, could also incorporate into and cause mutations in human DNA.The study authorsconclude that ‘’mutations in host DNA could potentially “contribute to the development of cancerand that the Molnupiravir metabolite the ribonucleoside analog β-D-N4 -hydroxycytidine (NHC), was a far more potent mutagen than favipiravir (FAV), which is a drug that has widely known issues related to teratogenicity and links to birth defects7.A recent article (preprint) also showed that invitro, Molnupiravir retains its activity against the VOCs Alpha, GHB-03021/2020* and Omicron. This is in accordance with the observation that the target proteins of these antivirals are highly conserved8.
This review aims to provide a summary of the available evidence from randomized clinical trials of Molnupiravir for treatment of COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 02/01/ 2022.
We searched the above databases and found 25 records. After removing duplicates and excluding that did not match our PICO question, 3 RCTs were included for meta-analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Hospitalized or death in 28 days
- Time to progression of each targeted COVID-19 sign/symptom
- Clinical improvement –as defined by 2-point decrease in the WHO clinical progression scale
- Important (secondary):
- Mortality (all-cause)
- Time to clinical improvement
- Time to viral clearance
- Adverse events: all and serious
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covid.nma database. If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
Results
We found 3RCTsthat matched the PICO question as pre-defined by the expert working group.
We found 3 RCTs that matched the PICO question as pre-defined by the expert working group.
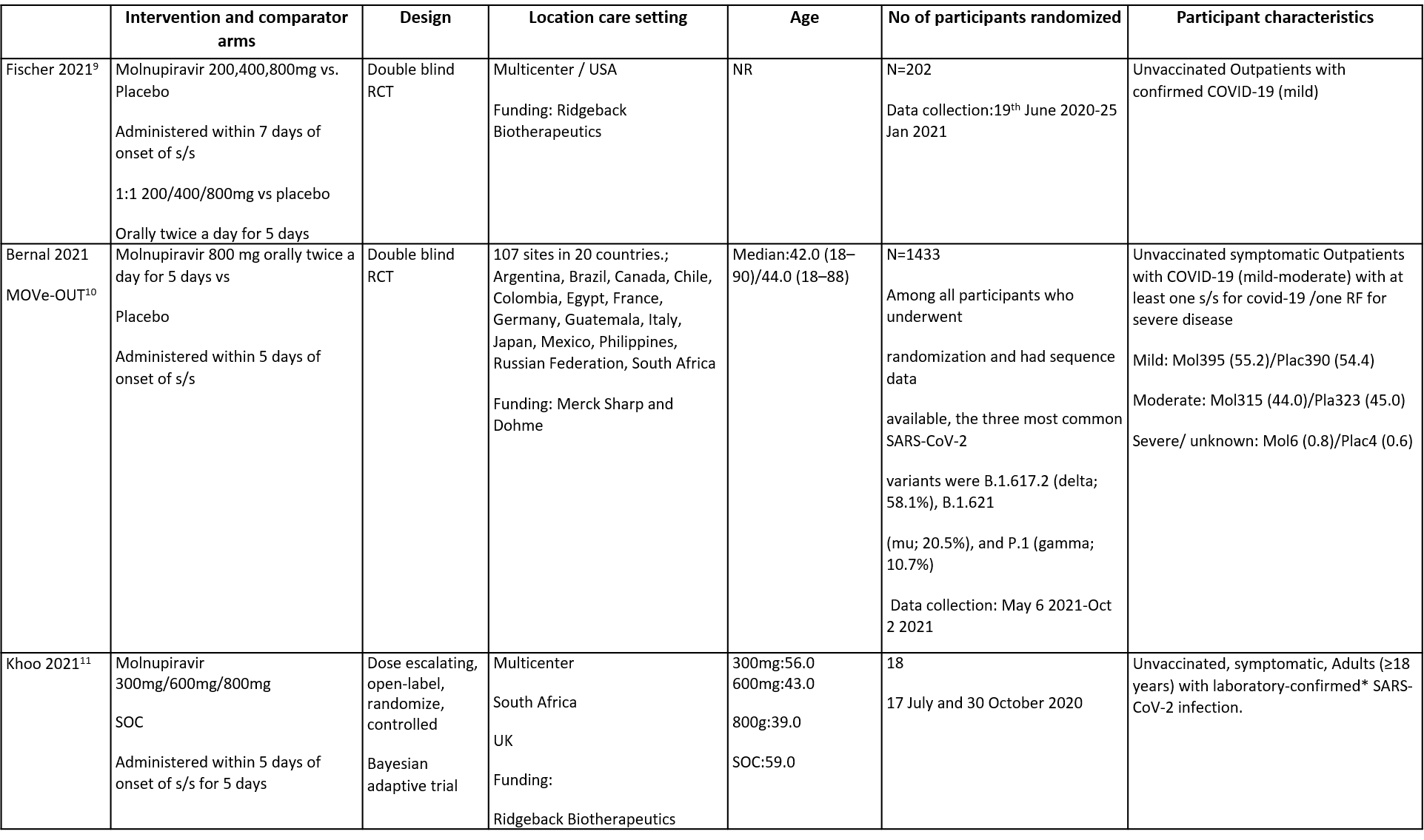
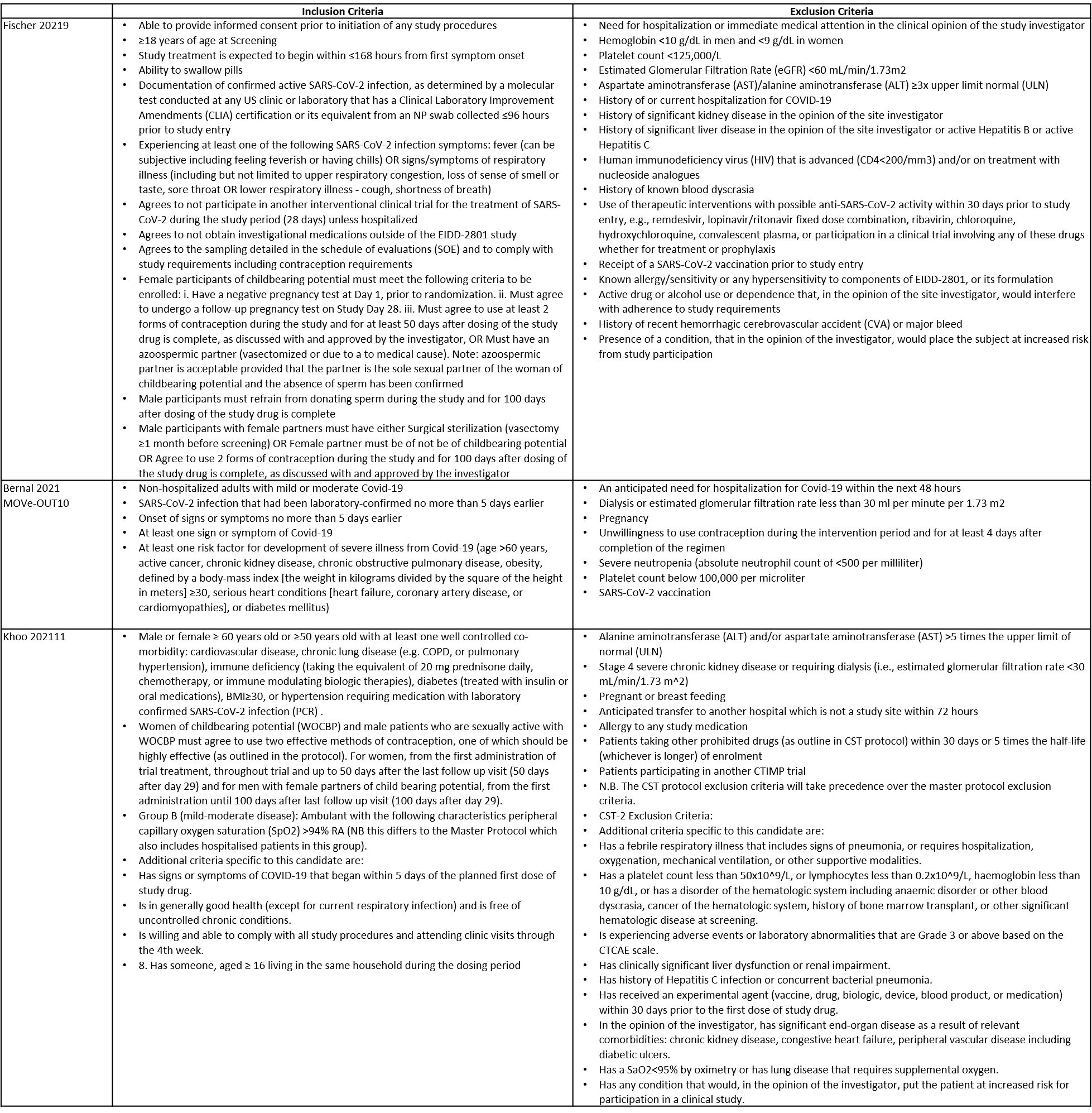
The trials included a total of 1653 participants, all of whom were adults9,10,11. One trial reported from United States of America9, other two are multicenter studies, one involving 107 sites in 20 countries10 and the other trial from UK and south Africa11. The first two trials9,10 compared Molnupiravir with Placebo whereas the third compared Molnupiravir with standard of care11. The trials recruited patients who were unvaccinated with mild or mild to moderate disease. Molnupiravir was administered within 5 days of symptom onset in two trials10,11 whereas in the other it was within 7 days9.
Each trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Favipiravir vs. standard of care or placebo).
● Two trials compared Molnupiravir with placebo (1635 participants).9,10
● One trial compared Molnupiravir with standard of care (18 participants).11
Our expert working group classified hospitalised or death in 28 days, time to progression of each targeted COVID-19 sign/symptom, clinical improvement –as defined by 2-point decrease in the WHO clinical progression scale as critical outcomes whereas mortality (all-cause), time to clinical improvement, time to viral clearance, Adverse events: all and serious as important outcomes.
Critical (primary) Outcomes
As presented in the ‘Summary of findings’ table, there is low or very low certainty evidence for effect of Molnupiravir on hospitalization or death at 28 days, all-cause mortality, incidence of viral PCR negativity D7, adverse events and serious adverse events. Clinical improvement scale 1-3(mild) to 0 (no symptoms) at Day 29 was found to have moderate quality evidence.
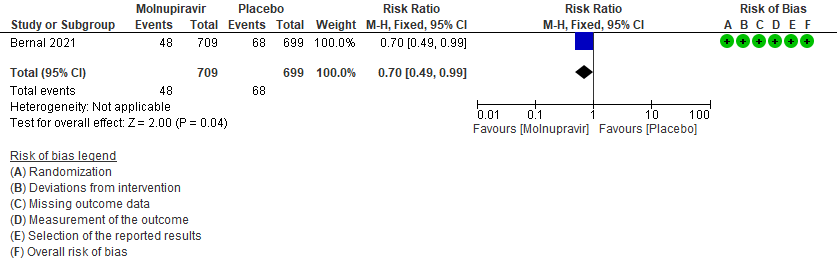
- Hospitalized or death in 28 days: Low certainty of evidence in 1408 patients in one RCT10 found that Molnupiravir may reduce hospitalization or death in 28 days compared to placebo (RR: 0.69; 95%CI: 0.49 to 0.99).
- All-cause mortality D28: Low certainty of evidence in 1610 patients in two RCTs9,10 found that Molnupiravir may reduce all-cause mortality vs. placebo (RR 0.12; 95% CI 0.02-0.68). However the event rate in this trial was very low in each of the groups.
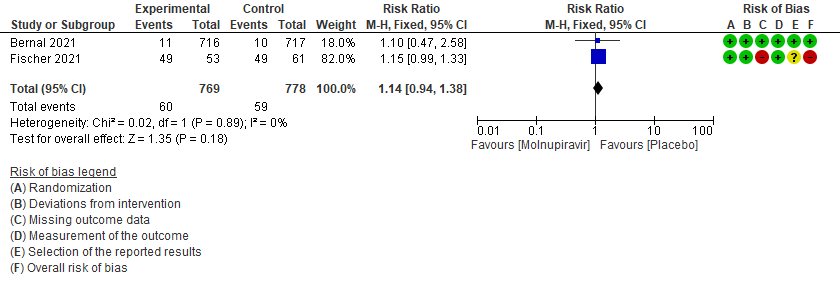
- Incidence of viral PCR negativity by D7: Very low certainty of evidence in 1547 patients in two RCTs9,10 found that the evidence is very uncertain about the effect of Molnupiravir on the incidence of viral PCR negativity by Day7 (RR 1.14; 95% CI 0.94-1.38).
- Clinical improvement by WHO progression scale 1-3 to 0: Moderate certainty of evidence in 1295 patients in one RCT10 found that Molnupiravir probably makes no difference to clinical improvement by Day 29(RR 1; 95% CI 0.89-1.12).
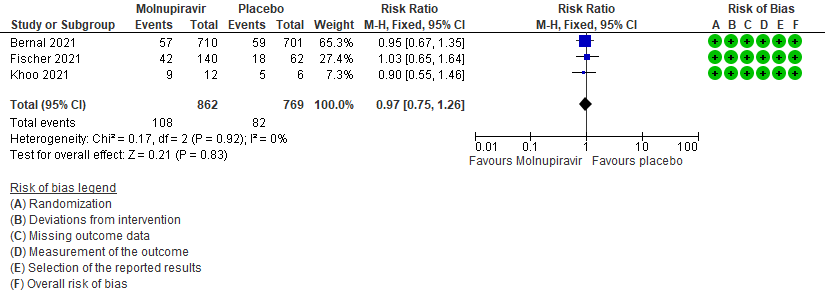
- Adverse events: Low certainty of evidence in 1631 patients in three RCTs9-11 found that Molnupiravir may result in little to no difference in overall adverse events (RR 0.97; 95% CI 0.75-1.26).
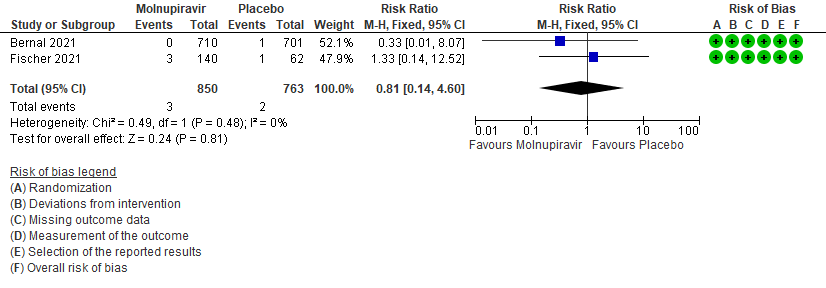
- Serious Adverse events: Very low certainty of evidence in 1613 patients in two RCTs9,10 found that the evidence is very uncertain about the effect of Molnupiravir on serious adverse events (RR 0.81; 95% CI 0.14-4.60).
A subgroup analysis was attempted as suggested by the Methodology group on a few outcomes based on the observations made by the experts.
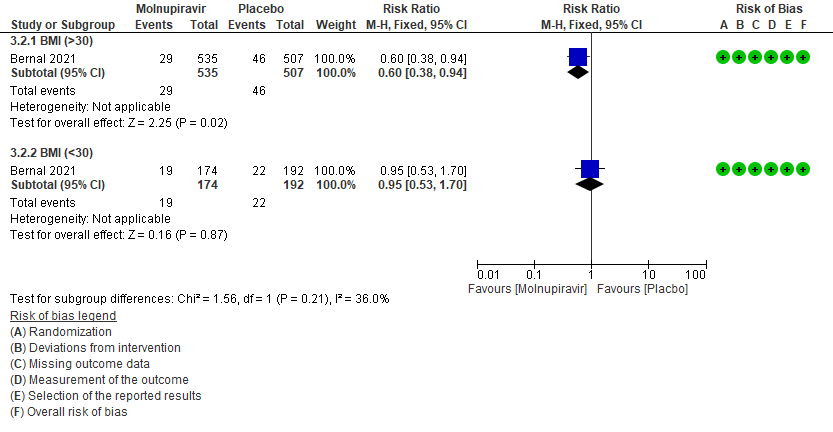
a. Incidence of hospitalization or death at day 29 by subgroup BMI>30
In the Bernal 2021/MOVe-OUT trial10 75% of the patient population were obese. And since obesity is considered as a risk factor for progression to severe disease, a subgroup analysis was attempted in patients with BMI >30 and in those with BMI 30 by 40% (RR:0.60;95% CI 0.38-0.9).
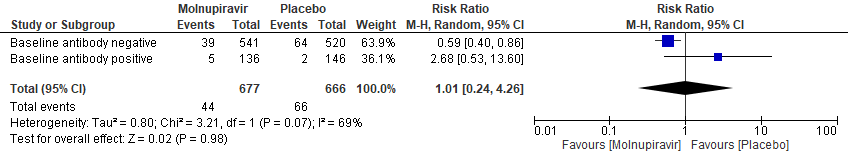
b. Incidence of hospitalization or death at day 29 (MITT) Subgroup analysis by baseline SARS-CoV2 nucleocapsid antibody status(suggesting previous infection)
In the Bernal 2021/MOVe-OUT trial10, Molnupiravir seemed to have a better response in those who were antibody negative at baseline. However, this was an observation made from available data in very few patients (RR: 0.59; 95% CI 0.40-0.86).
c. Incidence of hospitalization or death at day 29(mitt) baseline clade variant
In the Bernal 2021/MOVe-OUT trial10, among all participants who underwent randomization and had sequence data available, the three most common SARS-CoV-2 variants were B.1.617.2 (delta; 58.1%), B.1.621(mu; 20.5%), and P.1 (gamma; 10.7%). Molnupiravir did not show any differential benefit in any of the variants included in the subgroup analysis.The final effect estimate for this outcome is unknown, pending completion of all baseline sequencing; the current effect size may be an over or under-estimate. “Other” includes the following clades: 19B, 20A, 20B, 20C, 20D, and unknown clades or those that could not be classified.
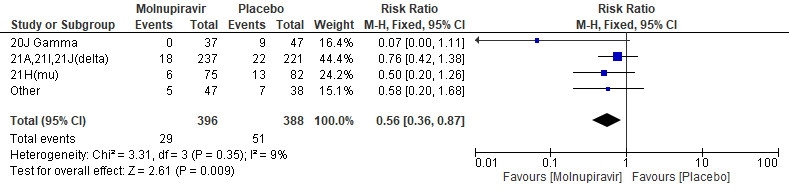
Primary:
1. Hospitalization or death at 28 days10
2. Time to progression of each targeted COVID-19 sign/symptom- Bernal 202110
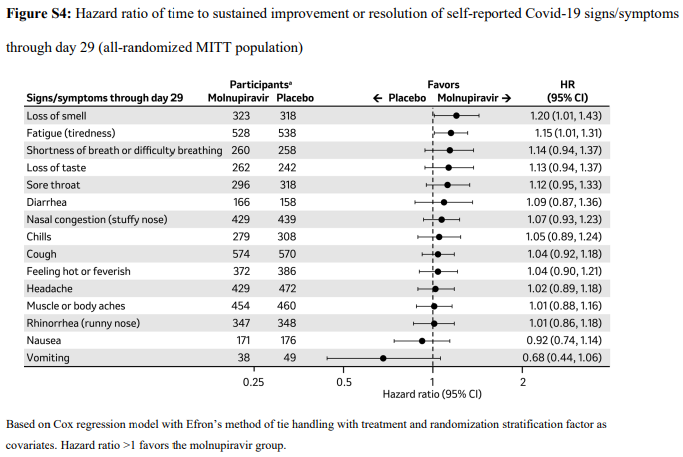
3. Clinical improvement –as defined by 2-point decrease in the WHO clinical progression scale10. Since most of the patients were classified as mild to moderate, at baseline they were at scale 1-3 (mild symptoms). For clinical improvement we have tracked those who moved from WHO progression scale 1-3 to 0.
3 (a): Clinical improvement: Those who moved from WHO progression scale 1-3 to 0.
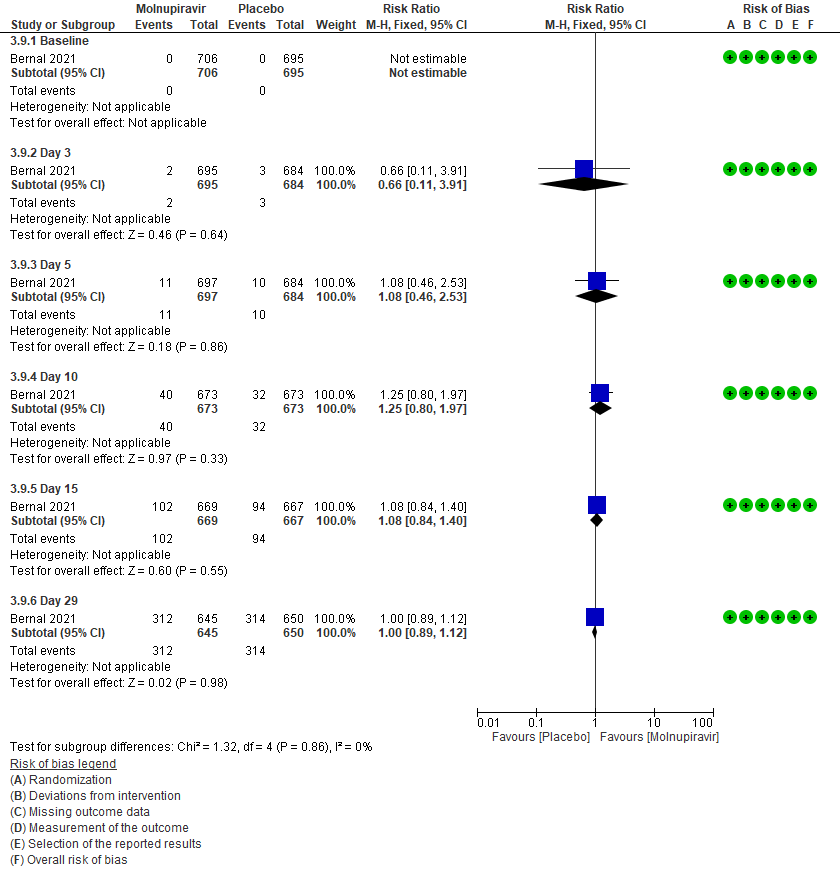
3 (b) Clinical worsening: Those who moved WHO progression scale 1-3 at baseline to > 3 at different time points
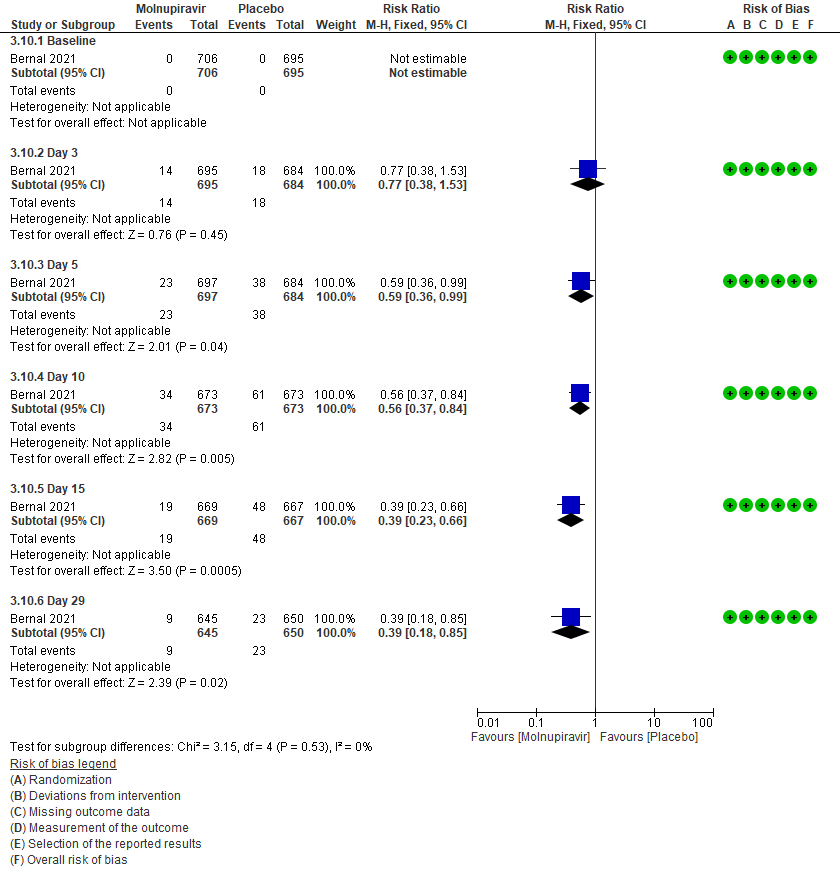
Secondary outcomes:
4. Mortality (all-cause)9,10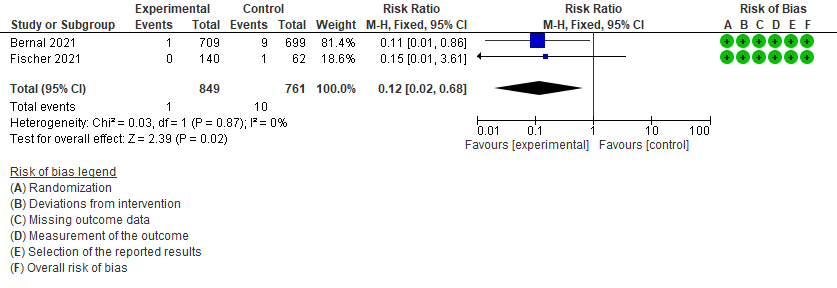
5. Incidence of viral PCR negativity at D79,10
6. Adverse events: All9,10,11
7. Adverse events: serious9,10
The Antiviral Expert Working Group met on 11th January 2022 to consider Molnupiravir as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Molnupiravir.
Summary of Judgments
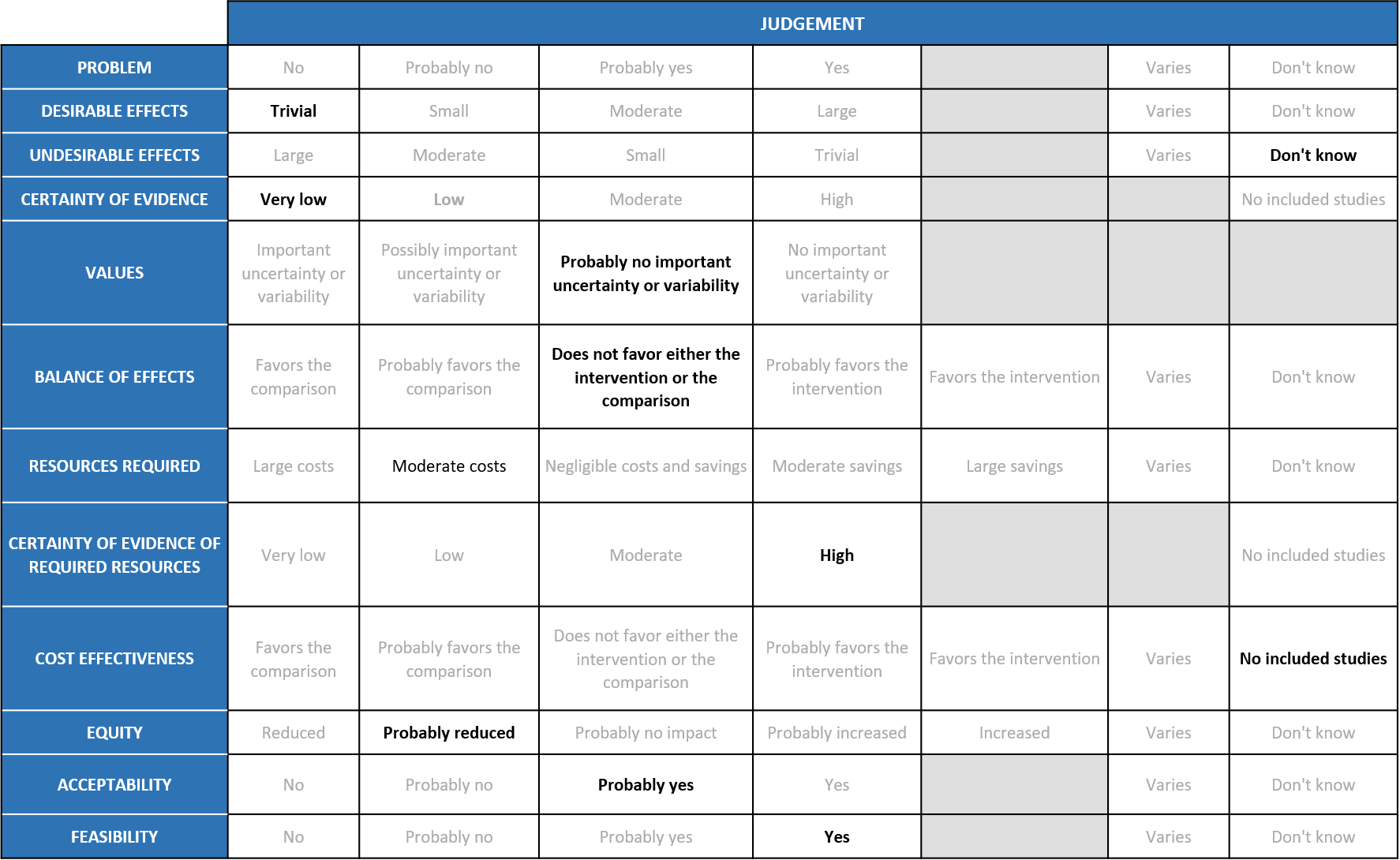
Problem
COVID-19 pandemic caused by the SARS-COV2 virus has significantly impacted India’s health structure by increasing the morbidity and mortality. Even though around 75% of the population is currently fully or partially vaccinated, an effective antiviral to treat or prevent COVID-19 is lacking. In the recent past, various drugs like Remdesivir were given emergency use authorization to treat COVID-19, but an oral antiviral agent specifically targeting mild disease population who doesn’t require hospitalisation was unavailable. Molnupiravir, being an oral pill was hence given emergency use authorization since an interim result from its manufacturer Merck’s study had shown significant reduction in hospitalisation or death in out-patients with mild covid-19. Since then, it is widely being in certain parts of the country for out-patients with mild disease with risk factors for severe disease. The group judged the problem to be of utmost priority.
Desirable effects
The group noted that in all the studies, participants were unvaccinated and treatment was started within 5 days of a positive PCR, so effects may be attenuated in current context. According to the data from studies, Molnupiravir has a slight effect in reducing hospitalization or death at 28 days RR 0.70 (95% CI 0.48 to 0.99) and all-cause Mortality RR 0.12 (95% CI 0.02 to 0.68) and no significant effect on conversion to negative PCR at Day 7 RR 1.14 (95% CI 0.94 to 1.38). Risk reduction of 30% may not translate to a high absolute risk with omicron variant of COVID-19 which causes predominantly mild disease or in the present scenario where most of the population is vaccinated. The group voted that the overall desirable effects are trivial. The mortality effects may seem large but it is based on a single trial with very low rate of events which is likely to over-estimate the effects.
Undesirable effects
The group agreed that according to the pooled data, Molnupiravir does not cause any significant adverse events RR 0.97 (95% CI 0.15 to 1.26) or serious adverse events RR 0.81 (95% CI 0.14 to 4.60). Since the data available is from patient population with mild to moderate disease, the group agreed that the sample size is inadequate to rule out serious adverse events. Also, long term serious adverse events like teratogenicity, mutagenicity and muscle damage were not explored in any of the studies included in the meta-analysis. Hence, the group agreed that they ‘don’t know’ about the undesirable effects of the drug.
Certainty of Evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence as low for hospitalised or death at Day 29, all-cause mortality D28 and adverse events. Evidence was very low for Incidence of viral negative conversion by D7 and serious adverse events. There was moderate certainty of evidence for Clinical improvement scale 1-3 to 0 Day 29. The expert working agreed with these judgements and rated the overall certainty of evidence as very low.
Values
The EWG felt that there is probably no important uncertainty or variability as to how the outcomes were valued. The group felt that all the outcomes were expressed variably in the studies and that time to negative PCR is not a clinically relevant outcome. Hospitalization or death at Day 29 and all cause mortality at Day 29 were the only outcomes that favored Molnupiravir over placebo/SOC. Though the certainty of evidence was low regarding the effect estimate, the group agreed that stakeholders (for e.g., individual patient, health care providers, public or private health systems etc.) may value the beneficial effect of Molnupiravir on these outcomes differently.
Balance of effects
The expert working group felt that the balance of effects does not favor either the intervention or the comparison. The panel felt that there is no convincing evidence to prove the benefit of this intervention. Since the studies have looked at unvaccinated population with mild to moderate disease across the studies, the panel felt that it is not representative of the current scenario. The panel also agreed that in the current scenario where >70% of the Indian population is partially or fully vaccinated, the vaccine efficacy and previous infection may play a role.
Resources required
The group felt that the costs were moderate to deliver this intervention as this is an oral pill and the total costs for a single course is around Rs 1400.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The panel discussed and agreed that there was no research evidence that evaluated cost of Molnupiravir in an Indian context.
Equity
At this point this intervention would reduce equity as it is not freely available and the costs are around Rs 1400 which is considerable when used for a large number of people with mild disease in the outpatients.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policy-makers, patients and clinicians) as it is an oral drug and can be administered easily. The panel discussed that even though the drug is not included in the treatment guidelines for India, clinicians might decide to choose the drug based on multiple risk factors or in certain populations but not others –e.g., older patients less likely to be affected by teratogenicity concern, though less evidence for benefit in that subgroup so far.
Feasibility
This is a feasible intervention if found efficacious as it is of low cost and can easily be delivered in a short course of 5 days.
Due to risk of teratogenicity and predisposition to malignancies, Molnupiravir should not be given to women of child bearing age, or to children. As a relatively affordable oral drug, and one of few available for mild to moderate covid-19, it may be difficult to implement procedures to ensure it is reserved for selected patients, for example those at high risk of progression to severe disease. Clinicians, hospitals and policymakers should consider how to put appropriate measures in place to ensure patient safety. Additionally, as Molnupiravir is a new drug, caution should be taken to follow patients up closely, at least clinically, to allow rarer or unknown adverse effects to be detected.
In the randomized trial the study and control groups were unvaccinated adults. The benefits that were seen were only in obese individuals with a BMI > 30. There was no significant difference in the other sub-groups studied.
Concerns have been raised about potential teratogenicity and mutagenesis issues. Therefore, it is clearly not a drug to be used in pregnant women and the recommendation is to ensure that pregnancy is avoided for at least 3 months after the medication is given and women need to follow contraception for the period. There is also a recommendation that men need to take similar precautions for the same period and ensure that their partners do not become pregnant. Obviously, the drug is not for use in children in view of these safety concerns.
It is not clear how beneficial the drug is in a vaccinated population on the basis of the published study. As the drug is for early use, it is not evident from present data if it benefits those with multiple standard risk factors like diabetes, hypertension, obesity, coronary heart disease, old strokes which are common in the older Indian population. If the drug is to be used these and other elderly immunosuppressed patients is probably the group it needs to be considered in though data for it does not exist as of now.
The pros of the drug are that it is not very expensive in the Indian setting and conventional early side effects are not very prominent. It would be prudent to actively discourage prophylactic use and use in those with only URI symptoms. If at all it is considered, it would be in the elderly group with co-morbidities and systemic symptoms within 5 days of symptom onset. It would be prudent to get a formal informed consent prior to prescribing the drug.
Molnupiravir is an antiviral with an advantage of being an oral drug and thus can result in misuse. The current evidence reveals small to moderate benefit in mild disease in those who are unvaccinated and obese (BMI>30) and hence there may be a marginal benefit in this specific subgroup. At present the pooled data does not suggest significant short-term adverse events, the number studied for safety data maybe inadequate. Moreover, though this antiviral act on RdRp, it differs from remdesivir. While remdesivir causes chain termination of SARS-CoV-2 virus, Molnupiravir causes mutagenesis of the virus or ‘error catastrophe’ during transcription, thus killing the virus. A study published last year found that the Molnupiravir metabolite, the ribonucleoside analog β-D-N4 -hydroxycytidine (NHC), was a far more potent mutagen than favipiravir (FAV), and its relation to genotoxicity needs to be explored and monitored. This recommendation against the use of Molnupiravir will be reviewed as and when, any new evidence emerges.
The MOVE-out study recruited unvaccinated patients prior to the emergence of Omicron variant. Further research is needed for
1.Assessing treatment efficacy/effectiveness in fully vaccinated patients with mild illness and risk factors for progression
2. Assessing genotoxicity in more in-vitro and in-vivo models
3. Assessing the impact of Molnupiravir on inducing viral mutations that may have phenotypic consequences especially in immunocompromised individuals
4. Assessing the in vitro activity of Molnupiravir against the Omicron variant
- EIDD-2801 [Internet]. [cited 2022 Jan 13]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/145996610
- First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA [Internet]. GOV.UK. [cited 2022 Feb 7]. Available from: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra
- Commissioner O of the. Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults [Internet]. FDA. FDA; 2021 [cited 2022 Feb 7]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain
- Preliminary data indicate COVID-19 vaccines remain effective against severe disease and hospitalisation caused by the Omicron variant [Internet]. European Medicines Agency. 2022 [cited 2022 Feb 7]. Available from: https://www.ema.europa.eu/en/news/preliminary-data-indicate-covid-19-vaccines-remain-effective-against-severe-disease-hospitalisation
- Molnupiravir and Drug Development at Emory | Emory University | Atlanta GA [Internet]. [cited 2022 Jan 13]. Available from: https://news.emory.edu/tags/topic/molnupiravir/index.html
- Commissioner O of the. Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults [Internet]. FDA. FDA; 2021 [cited 2022 Jan 13]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain
- Huntai Et Al., The Journal of Infectious Diseases, Volume 224, Issue 3, 1 August 2021, Pages 415–419
- Vangeel L, Jonghe SD, Maes P, Slechten B, Raymenants J, André E, et al. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern [Internet]. 2021 Dec [cited 2022 Jan 13] p. 2021.12.27.474275. Available from: https://www.biorxiv.org/content/10.1101/2021.12.27.474275v1
- Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. Molnupiravir, an Oral Antiviral Treatment for COVID-19 [Internet]. 2021 Jun [cited 2022 Jan 15] p. 2021.06.17.21258639. Available from: https://www.medrxiv.org/content/10.1101/2021.06.17.21258639v1
- Bernal AJ, Silva MMG da, Musungaie DB, Kovalchuk E, Gonzalez A, Reyes VD, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. New England Journal of Medicine [Internet]. 2021 Dec 16 [cited 2022 Jan 15]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa2116044
- Khoo SH, Fitzgerald R, Fletcher T, Ewings S, Jaki T, Lyon R, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. Journal of Antimicrobial Chemotherapy. 2021 Dec 1;76(12):3286–95.
- Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BMD, Schinazi RF, Sheahan TP, Baric RS, Heise MT, Swanstrom R. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J Infect Dis. 2021 Aug 2;224(3):415-419. doi: 10.1093/infdis/jiab247. PMID: 33961695; PMCID: PMC8136050
Covid Management Guidelines India Group – Anti-Viral Working Group – Molnupiravir. Covid Guidelines India; Published online on February 16th, 2022; URL: Molnupiravir – Covid Guidelines India (indiacovidguidelines.org) ()
