This recommendation applies to acute COVID-19 infection without a suspected or confirmed thrombotic event. The intent of this recommendation is thromboprophylaxis. For suspected or confirmed thrombotic events please follow usual therapeutic protocols as per standard hospital practice.
Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: The group recommends use of therapeutic anticoagulation in patients admitted to hospital with moderate or severe COVID-19 (strong recommendation) and a conditional recommendation for therapeutic anticoagulation in critical COVID-19. We have insufficient evidence to recommend anticoagulation in non-severe COVID-19 illness.
DATE OF RECOMMENDATION: 08th August 2022
There is insufficient evidence for therapeutic or prophylactic anticoagulation in non-severe COVID-19 illness
A strong recommendation is one for which the panel is confident about the desirable effects over undesirable effects of the intervention, or other aspects, such as cost and feasibility.
The panel recommends a dose of LMWH 40 mg OD to LMWH 1 mg/Kg/OD SC, or Unfractionated Heparin 5000 U Q12H SC.
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
A strong recommendation is one for which the panel is confident about the desirable effects over undesirable effects of the intervention, or other aspects, such as cost and feasibility.
The panel recommends a dose of LMWH 1 mg/Kg/BD SC.
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
A conditional recommendation is one for which the panel is less confident about the balance between desirable and undesirable effects of the intervention, or other aspects, such as cost and feasibility.
The panel recommends a dose of LMWH 1 mg/Kg/BD SC.
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
Therapeutic anticoagulation probably reduces thrombosis and thrombosis-related death without increasing risk of fatal bleeding in patients admitted to hospital with moderate to severe COVID-19. While it does not have a mortality benefit in critical COVID-19 patients RR (1.02 95% Ci: 0.85 -1.22), it does reduce thrombosis without fatal bleeding in critical COVID-19 illness.
COVID-19 infection is associated with a hypercoagulable state. Thrombotic events, both venous and arterial thromboembolism have been noted. Therapeutic anticoagulation is a low-cost intervention and needs to be weighed against hospitalization and ICU care costs that may result due to a thrombotic event. Clinicians need to balance between the risk of thrombosis and the risk of bleeding in an individual patient.
With new Randomised Control trials (RCTs) available the group decided to update the recommendations based on a meta-analysis of 7 RCTs 1–7.
In the light of the present variant being Omicron, it was noted that most of the patients with mild illness require only ambulatory or domiciliary care. Data was insufficient to make a recommendation for therapeutic anticoagulation in this category as there has been only one Open label RCT looking at non-severe Covid. However, though these patients were in hospital they had only non-severe COVID. The group noted very low to low certainty and hence were uncertain about the effect of therapeutic anticoagulation on all-cause mortality and thrombotic events in non-severe COVID-19 illness. Hence the working group suggested prophylactic anticoagulation be based on clinical judgement of the treating physician.
Meta analysis noted that therapeutic anticoagulation in moderate to severe cases of COVID-19 probably decreased thrombosis and thrombosis related death as compared to non-therapeutic anticoagulation [moderate certainty of evidence, [RR 0.46 (95% CI 0.27 - 0.80)] and [RR 0.62 (95% CI 0.39-1)] respectively. There was a slight increase in survival in patients without organ support. [moderate certainty of evidence, [RR 1.05 (95% CI 1.01-1.10)]. It was noted that there is increased risk of major bleeding in the therapeutic anticoagulation group [RR 1.78, 95% CI (1.00-3.15)], however only 5 cases of fatal bleeds were noted from 7 trials with 4747 patients, of which 4 are in the therapeutic anticoagulation group and 1 is in the prophylactic anticoagulation group. Based on this the working group made a strong recommendation for the use of therapeutic anticoagulation in moderate to severe Covid 19.
In Critical COVID-19 patients, therapeutic anticoagulation caused little or no difference in all-cause mortality or thrombosis related deaths compared to non-therapeutic anticoagulation group. There was no appreciable difference in organ support free days compared to non-therapeutic group [OR 0.86, 95%CI (0.7-1.06)]. Therapeutic dose anticoagulation probably does reduce thrombosis in critical covid-19 patients {moderate certainty of evidence, [RR 0.65 (95% CI 0.46-0.92)]}. The group discussed in detail about the pros and cons of therapeutic anticoagulation in this category of severity and made a conditional recommendation for therapeutic anticoagulation.
Date of latest search: 14 October 2021
Date of completion of Summary of findings table and presentation to Expert Working Group: 02 April 2022
Evidence synthesis team: Sushil S(SS), Jisha Sara John (JS), Richard Kirubakaran, Bhagteshwar Singh & Priscilla Rupali.
We acknowledge gratefully the assistance received from the authors of the multi-platform RCT [mpRCT] (ATTACC, ACTIV-4a, and REMAP-CAP platforms), specifically Ewan Goligher for sharing additional protocol documents, and Alexander Spyropoulous of the Hep-Covid trial who provided valuable assistance in evidence clarification.
Overall
Explanations
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In Marcos et al1. Domain 1 was marked down for some concerns due to inadequacy in allocation concealments. In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes. In HESACOVID 3 trial, Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' . In Marcos et al1 Domain 1 was marked down for some concerns due to inadequacy in allocation concealments. In 2 domains of the mpRCT studies4,5 'some concerns' were identified. Regarding Domain 2 see explanation (a). Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from a clinically unimportant harm to appreciable harm.
e. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
1. Outcomes in the WHO mild category of patients
Explanations
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes.
b. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns’. in 2 domains of the mpRCT4,5 Re Domain 2 see explanation (a). Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
e. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
f. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically insignificant benefit to appreciable benefit.
2. Outcomes in the WHO moderate (on oxygen) and severe category of patients
Explanations
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes.
b. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns’. in 2 domains of the mpRCT4,5 Re Domain 2 see explanation (a). Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
e. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
f. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically insignificant benefit to appreciable benefit.
3. Outcomes in critical category of patients
Explanations
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes. In HESACOVID3 trial, Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious imprecision; 95% CI ranges from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains for mpRCT (Zarychanski et al4.), and in one domain for other trials, for measurement of this outcome. In the mpRCT (Zarychanski et al.)4, Domain 2 was marked down for 'some concerns' - see explanation a. Domain 4 was assessed to have 'some concerns' because of the open-labelled nature of the trials which may have impacted aspects of assessment of this outcome. In HESACOVID trial3, Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
d. Not downgraded for imprecision as, even at the upper 95% CI, the benefit was considered clinically significant.
e. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
Since December 2019, a worldwide pandemic labelled COVID-19, caused by SARS-CoV-2 virus has adversely impacted humanity in diverse ways. Clinical studies of hospitalized patients with SARS-CoV-2 initially showed flu-like symptoms, most commonly cough, sore throat, fever, myalgia, and fatigue at onset of COVID-19 illness, which could then develop into a viral pneumonia with varying severity 8. Abnormal coagulation profiles and thrombotic complications, both venous and arterial, are common among the hospitalized severe and critically ill patients 9, with pulmonary embolism being the most common site 10. Multiple autopsy reports show unprecedented pulmonary microvascular thrombosis and endothelial damage 11 which could be related to the direct viral cytopathic effect on the endothelial cells due to shared receptors with the alveolar cells 12. Other etiopathogenetic mechanisms include immune/cytokine mediated dysregulation of pro-coagulant & anti-fibrinolytic pathways.
Though hypercoagulability in COVID-19 has now been well-recognized, uncertainty still exists as to how best to manage clotting risk in these patients. In addition, an increased risk of hyper-coagulability leading to thrombotic events have been reported and recognized extensively in the media and among peers. There is a prevailing assumption that the delta variant in India may be contributing to an increased number of thrombotic events, however this needs to be systematically studied and documented. Over the past 2 year several guidance documents have recommended the use of anticoagulation in hospitalized patients with COVID-19 13–15. Most of these guidelines recommend the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) but whether to use at a therapeutic or a non-therapeutic dose remains unclear.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE, Epistemonikos (COVID Living Overview of Evidence platform), and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 14th October 2021. We also reviewed reference lists of systematic reviews and included studies.
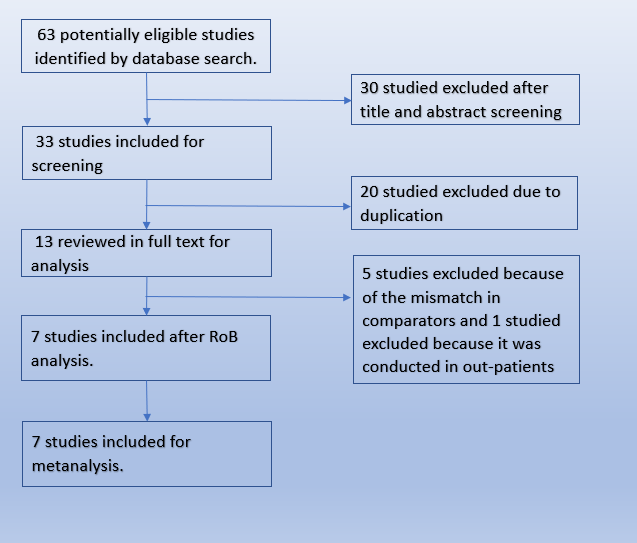
We searched the Pubmed database and found 63 potentially eligible records. After removing duplicates, and excluding studies that were not RCTs or did not involve the intended intervention, we found 7 trials that matched our PICO.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 21-30 days, or in-hospital
- Thrombotic events
- Important (secondary):
- Time to clinical improvement
- Organ support free days (OSFD): organ support defined as ventilator or dialysis or inotropic requirements.
- Survival without organ support at day 28
- Duration of hospitalization
- Bleeding events
Two reviewers (SS & JSJ) independently assessed eligibility of search results. One reviewer extracted data from each included study, and both assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool.
We used RevMan 5.4 to perform meta‐analysis using fixed & random‐effect models for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). Since the guidelines were going to be specific to each severity category, we grouped studies as per their inclusion criteria into their severity categories and combined outcomes to provide pooled estimates. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
Varied dosing strategies are used for anticoagulation depending on indication, organ dysfunction, BMI and adverse drug reactions, based on available literature and package insert recommendations. For purposes of our guidelines in the expert working group meeting it was decided to broadly specify doses - low & intermediate doses of non-therapeutic anticoagulation as 1mg/kg IV once daily AND therapeutic dose anticoagulation as 1mg/kg twice daily.
| Product | Thromboprophylaxis dose | Therapeutic dose | |
| Low dose | Intermediate dose | ||
| Low Molecular Weight Heparin (LMWH) - Enoxaparin | 40 mg q24h
[BMI>40/Weight >120 Kg – dose increase to 40 mg q12h] |
1 mg/kg q24h | 1mg/kg q12h |
| Unfractionated Heparin (UFH) | 5000U q12h | 5000 U q8h
OR 7500 U q12h |
80 U/kg bolus followed by 18 U/kg/hour infusion
Targeting APTT of 55-75 seconds |
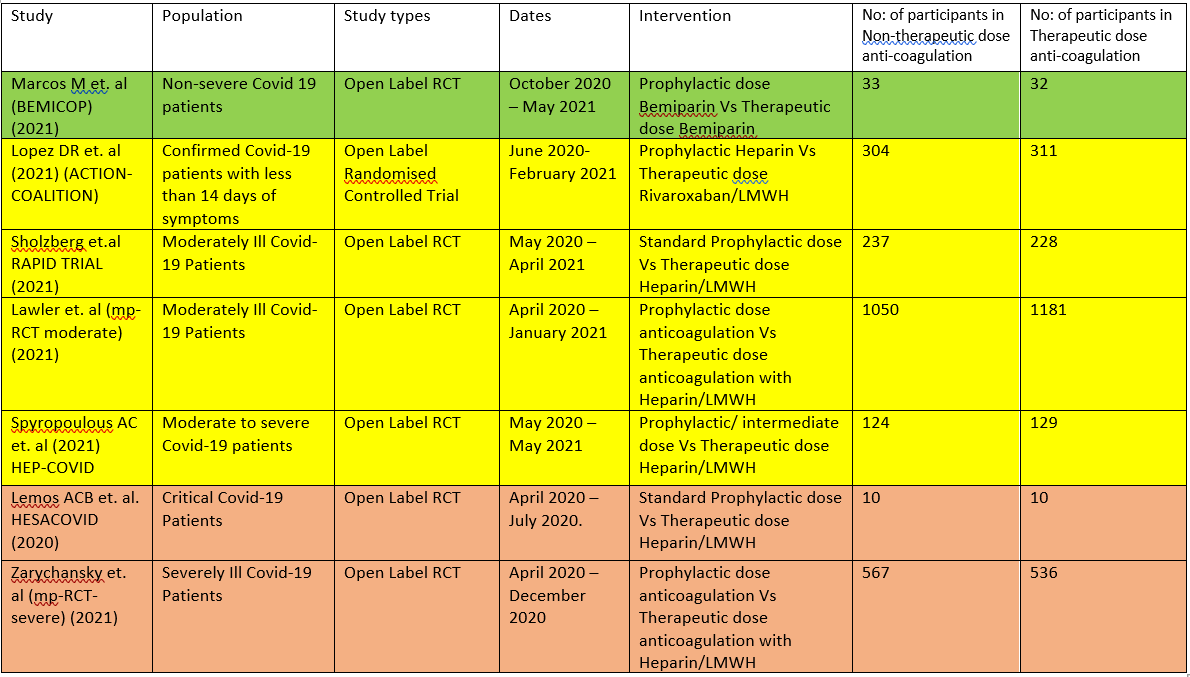
The 7 RCTS compare Therapeutic dose anticoagulation with non-therapeutic doses:
- HESACOVID 3:- compared prophylactic vs therapeutic anti-coagulation (20 participants).
- mpRCT(Critically ill) 4: Zarychanski et al. is a collaboration of 3 RCT platforms, ACTIV-4a, REMAP-CAP and ATTACC, looking specifically at critically ill patients (total of 1074 participants) – comparing therapeutic vs non-therapeutic anticoagulation.
- mpRCT(Non-critically ill) 5:- Lawler et al. is a collaboration of above mentioned 3 RCT platforms, looking specifically at non-critically ill hospitalized patients (total of 2291 participants) – comparing therapeutic vs non-therapeutic dose of anticoagulation.
- COALITION- ACTION 2: Looks specifically at the moderate-severe category of patients. In this trial, severe patients were administered LMWH, while moderately ill patients were administered Rivaroxaban, which is a directly acting Factor Xa inhibitor.
- RAPID Trial 6:- It compared prophylactic dose of anticoagulation Vs Therapeutic dose of anticoagulation in moderate to severe ill patients
- HEP-COVID Trial7 :- Therapeutic Vs. non-therapeutic dose of anticoagulation in moderate to critically ill patients.
- BEMICOP Trial 1:- Prophylactic Vs. Therapeutic dose of Bemiparin is compared in mild Covid-19 patients.
The critical outcomes that were available and extracted for analysis from these studies included
- All-cause mortality (21-30 days),
- Thrombosis,
- Organ support free days [i.e., surviving to hospital discharge with reduced need for ICU-level organ support, including invasive and non-invasive mechanical ventilation]
- Survival without organ support at 28 days
- Major bleeding events.
Though we are using the term non-therapeutic anticoagulation, the intent in all these trials was prophylaxis. Two different doses are being compared in each of these trials, therapeutic dose anticoagulation vs nontherapeutic dose (prophylactic or intermediate doses).
We included 7 trials1–7 with 4741 patients of which 1 trial did not contribute much data 3. The patients were compared across different pre specified COVID-19 severity groups, for the different outcomes as mentioned above (See summary of characteristics tables). The duration of administration of anticoagulation varied from a minimum of 96 hours to 14 days or till the patient got better.
We excluded 2 trials INSPIRATION 16 and Perepu17 et. al from our analysis as they are comparing prophylactic dose of anticoagulation with intermediate dose of anticoagulation with the intent here being “prophylaxis” rather than treatment.
The group of patients studied in the mpRCT (Critically ill) study, correlated directly with WHO severity criteria of critical category. The group of patients studied in the mpRCT (non-Critically ill) study, correlated with WHO severe category also overlapping with a few in the WHO moderate category. The population in the Hep-covid study 7 included “moderate to critical” category of patients. Hence, when performed a sub-group analysis we stratified data obtained from authors and analysed moderate to severe patients separately and critical patients separately.
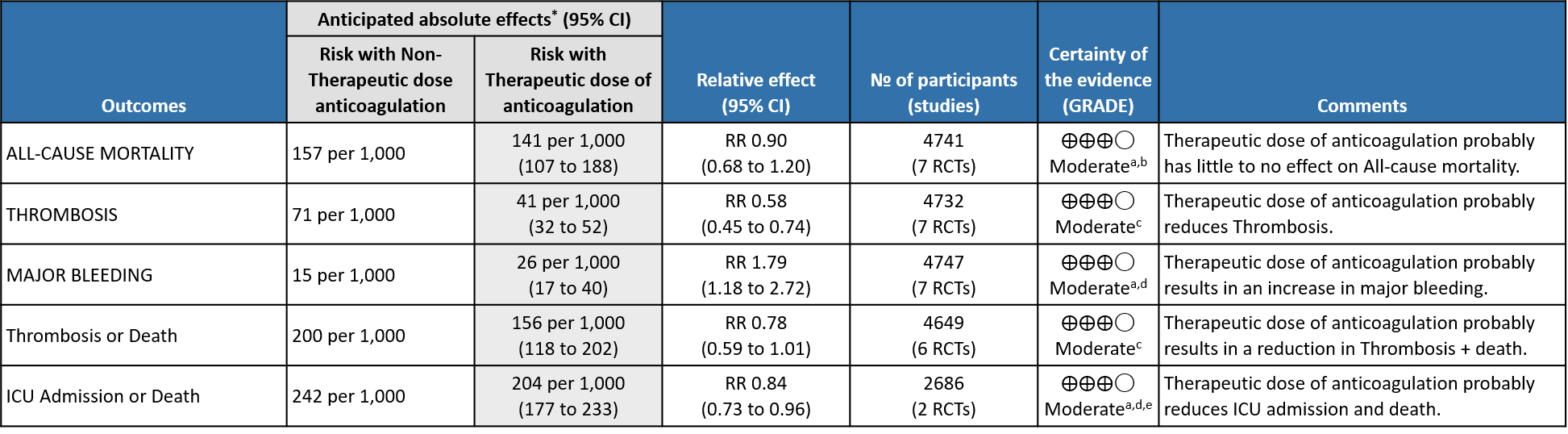
Overall analysis: Using GRADE methodology, certainty of evidence is shown in Summary of Findings tables.
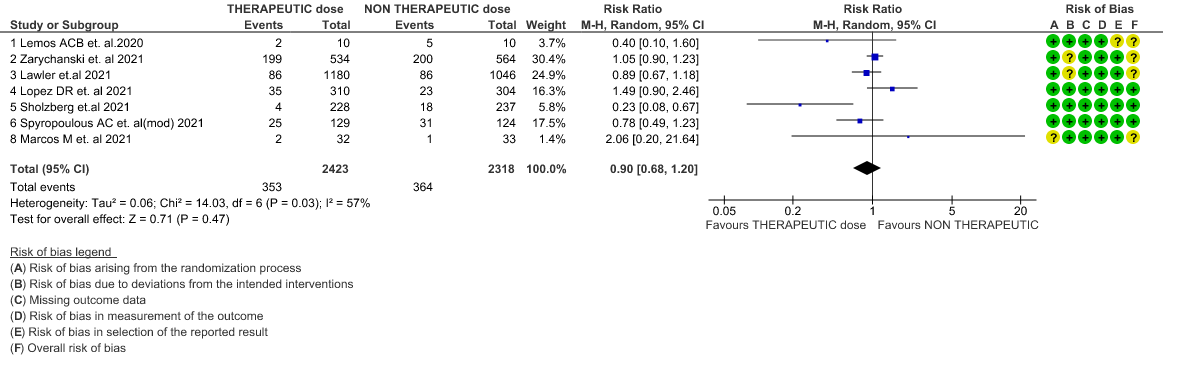
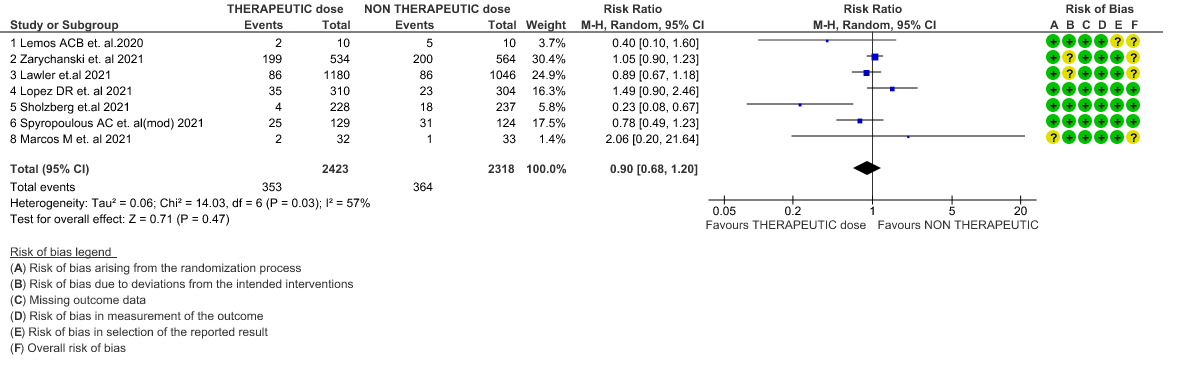
- All-cause Mortality- Moderate certainty of evidence in 7 RCTs1–7 with 4741 patients, revealed that therapeutic anticoagulation possibly results in little to no difference in all-cause mortality, compared with non-therapeutic anticoagulation (RR 0.90, 95 % CI=0.68 to 1.20).
- Thrombosis- Moderate certainty of evidence in 7 RCTs1–7 with 4732 patients, revealed that therapeutic anticoagulation probably reduces thrombosis, when compared with non-therapeutic anticoagulation (RR= 58, 95 % CI = 0.45 to 0.74).
- Major bleeding- Moderate certainty of evidence in 7 RCTs 1–7with 4747 patients, revealed that therapeutic anticoagulation probably results in an increase in major bleeding, compared with non-therapeutic anticoagulation (RR 1.79, 95% CI = 1.18 to 2.72).
- Organ support free days:- Evidence for OSFD data was compiled from 4 studies 3–6 and this outcome was variably expressed as mean ±SD, median with IQR, Odds ratio or adjusted Odd’s ratio. As the data cannot be pooled, we have represented the data in a table and no meta-analysis was done for OSFD. 2 studies5,6 that included moderate-severely ill patients showed that therapeutic anticoagulation gives more organ support free days than non-therapeutic anti-coagulation. Critically ill category of patients had data available from 2 trial 1 trial with 1103 patients 4 with critical category of patients showed no difference in OSFD with therapeutic or non-therapeutic anti-coagulation and 1 trial with only 20 participants 3 with critically ill patients showed significant benefit in OSFD, but had only 20 participants.
- Thrombosis or Death (composite outcome) :- Moderate certainty of evidence in 6 RCTs 2–7 with 4649 patients suggested that therapeutic anticoagulation probably reduces thrombosis or death in COVID-19 patients [RR : 0.78, 95% CI : 0.59 to 1.01].
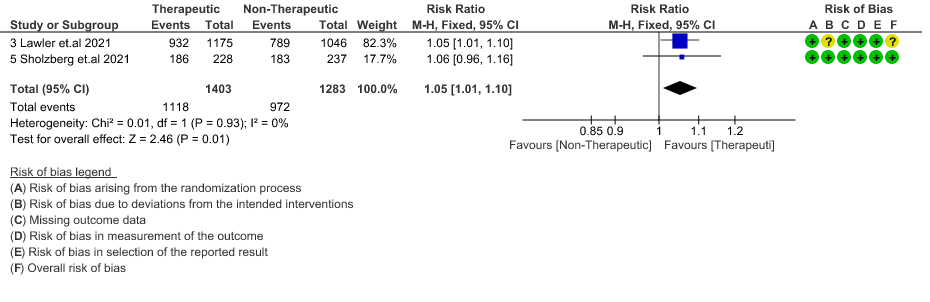
- ICU admission or death: - Moderate certainty of evidence in 2 RCTs5,6 with 2686 patients suggested that the therapeutic anti-coagulation probably reduces [RR : 0.84, 95% CI : 0.73 to 0.96] the need of ICU admission or death in moderate to severely ill patients.
Since guidelines are to provide recommendations for each severity strata, disaggregated data was also assessed to provide analysis to help formulate recommendations specific to each severity strata.
Analysis according to severity strata
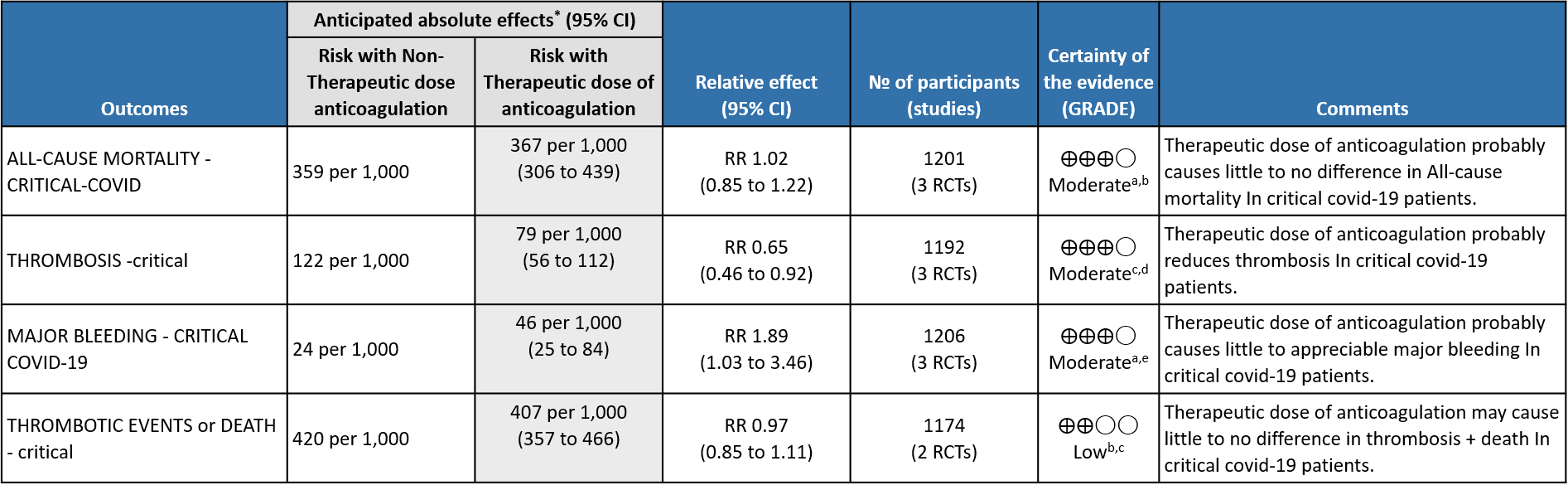
WHO Critical group: Moderate certainty evidence from 3 RCTs3,4,7 in 1201 patients showed that therapeutic anticoagulation results in little to no difference to all-cause mortality in critical COVID-19 illness (RR-1.02, 95 % CI = 0.85 to 1.22). However, moderate certainty evidence did show decreased thrombosis in patients (RR 0.65, 95 % CI = 0.46 to 0.92) but with an increase in bleeding (RR 1.89, 95 % CI = 1.03 to 3.46). In addition, evidence from 1 trial 4, with a larger group (1103) of patients, showed that therapeutic anticoagulation did not improve days free from organ support compared to prophylactic anticoagulation [Odds Ratio (OR) 0.86, 95% CI 0.70 to 1.06].
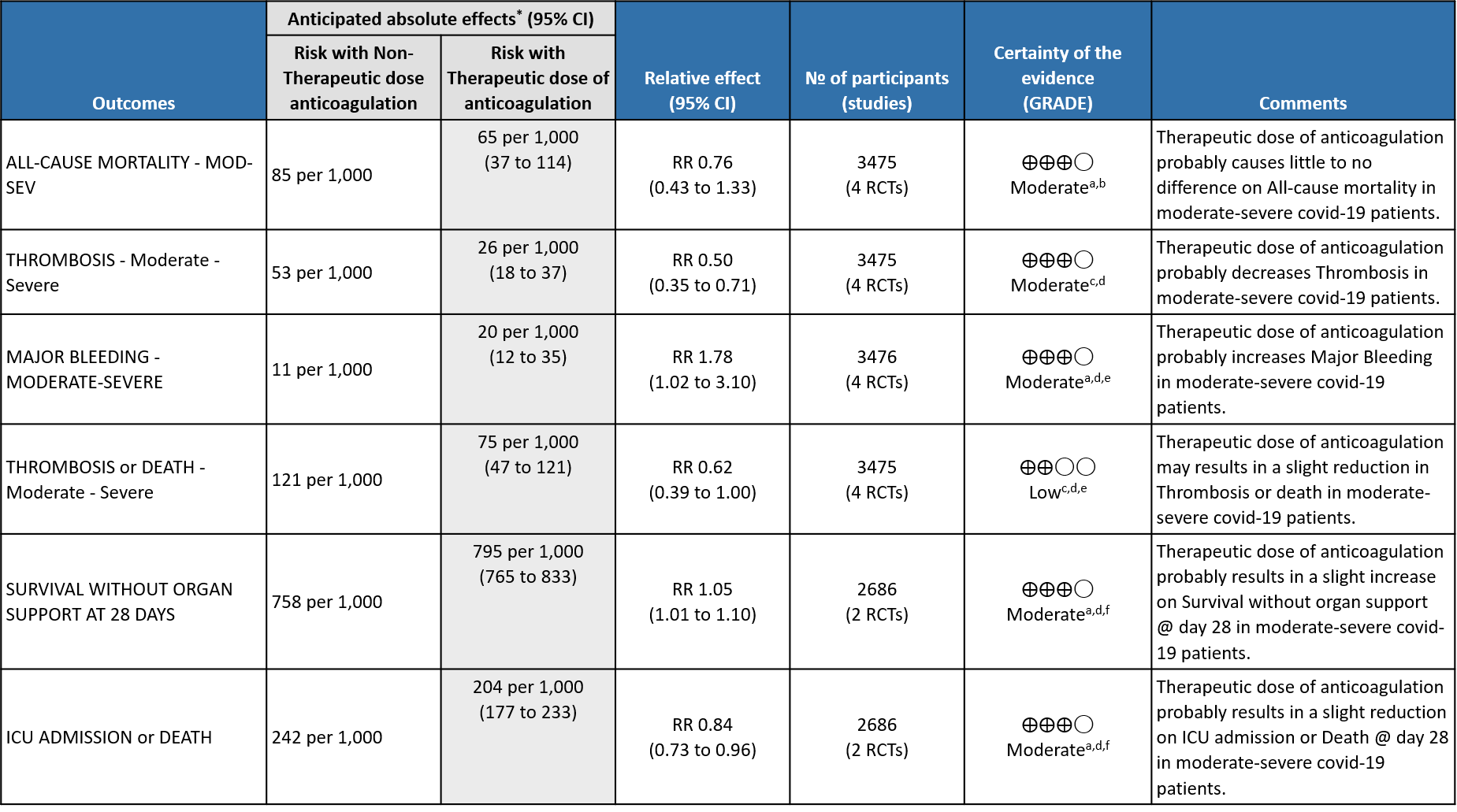
WHO Moderate/Severe group: Moderate certainty evidence from 4 RCTs2,5–7 in 3475 patients suggested that therapeutic dose of anticoagulation provided little to no mortality benefit [RR 0.76 (95% CI 0.43 to 1.33)] but did prevent thrombosis by 50% (RR 0.50, 95 % CI 0.35 to 0.71) with an increased risk of major bleeding (RR 1.78, 95 % CI 1.02 to 3.10).
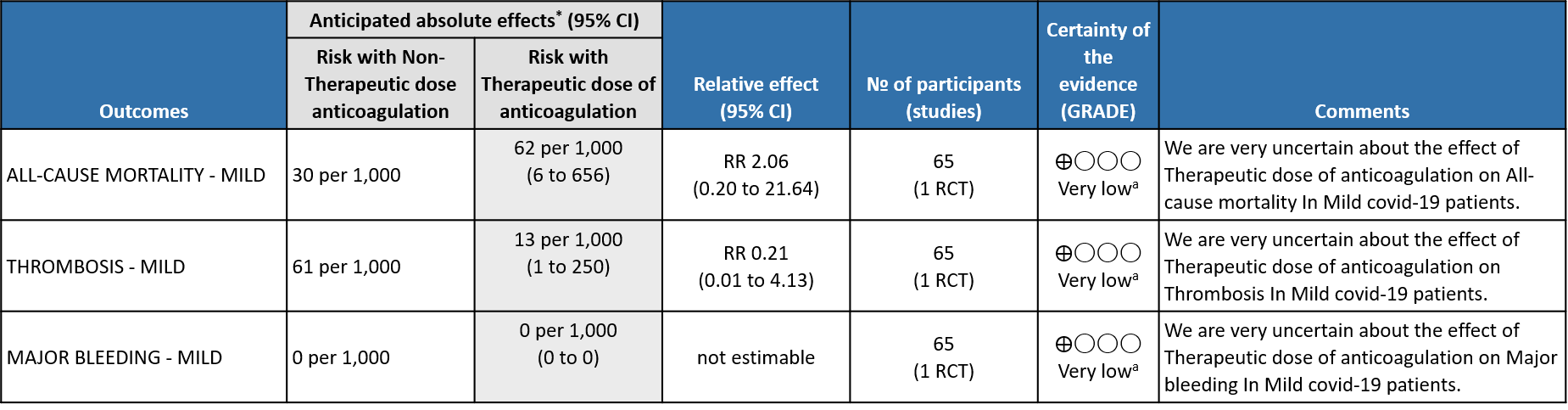
WHO mild group : Very low certainty of evidence from 1 RCT1 suggests that the effect of therapeutic anticoagulation was uncertain on all-cause mortality, thrombosis and major bleeding, in mild Covid-19 patients. One trial Activ-4b was excluded from the analysis as the study was conducted in an out-patient setting, which is not consistent with the definition laid out in our PICO. 18
Therapeutic vs Non-Therapeutic Dose of Anti-Coagulation
1. All-cause mortality (21-30 days)
2. All-cause mortality (21-30 days) (stratified according to the severity of disease)
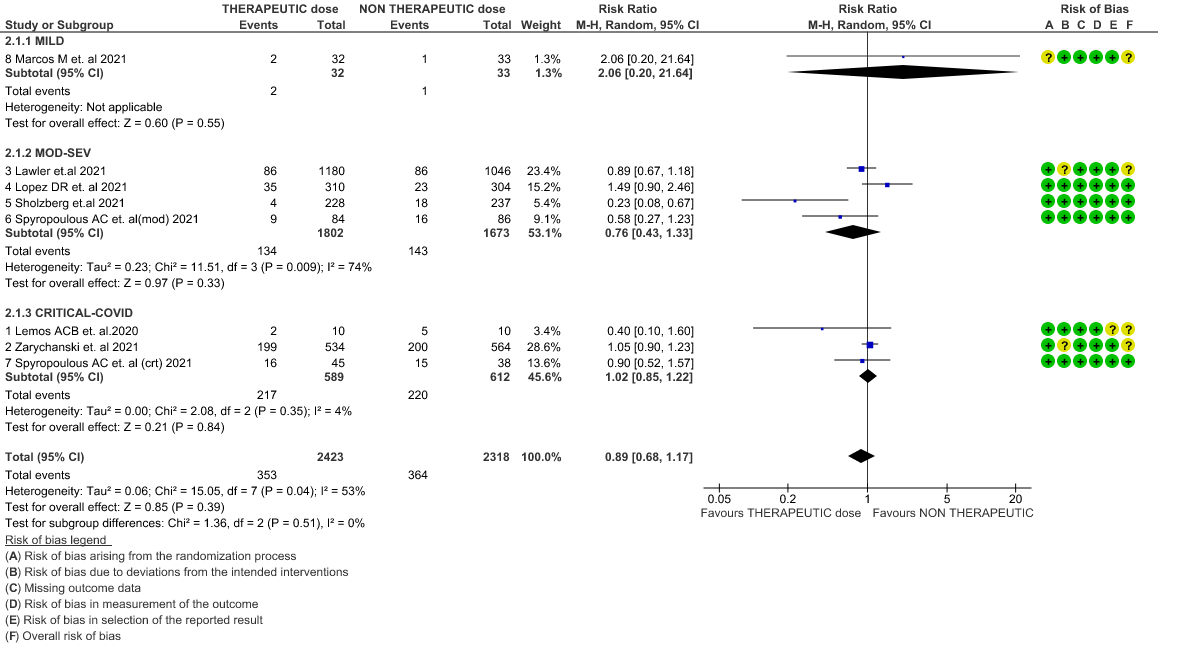
3. Thrombosis
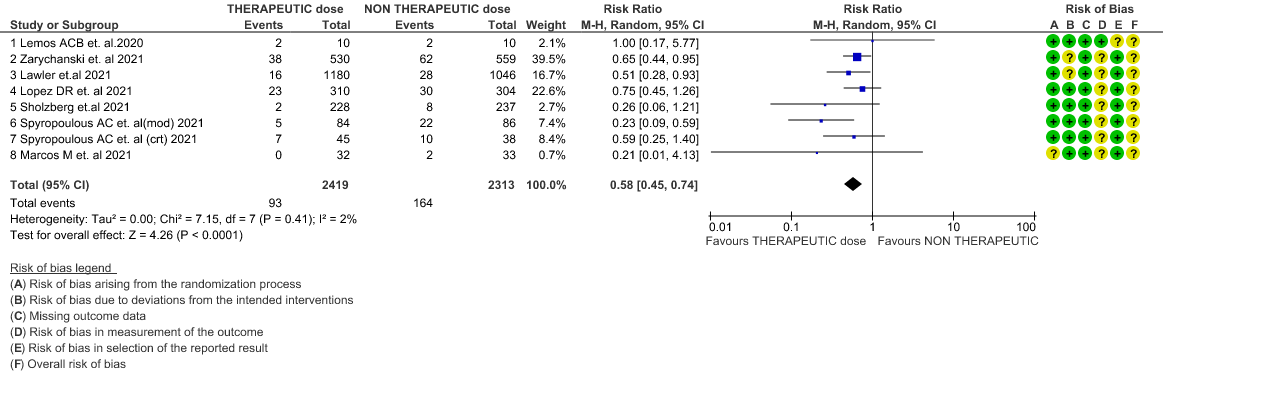
4. Thrombosis (stratified according to the severity of the disease)
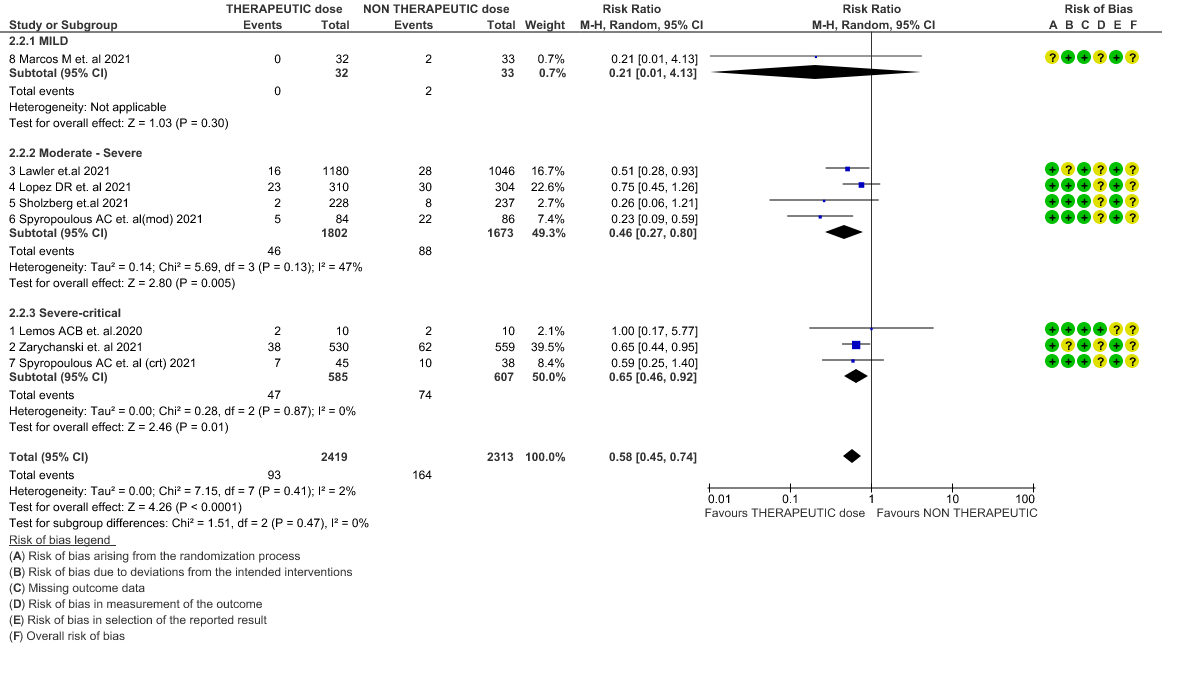
5. Major Bleeding
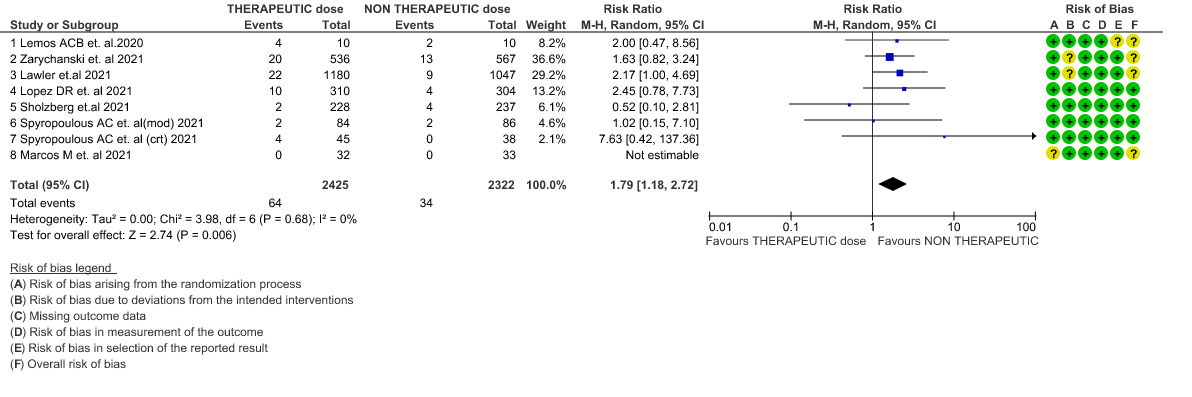
6. Major Bleeding (stratified according to the severity of the disease)
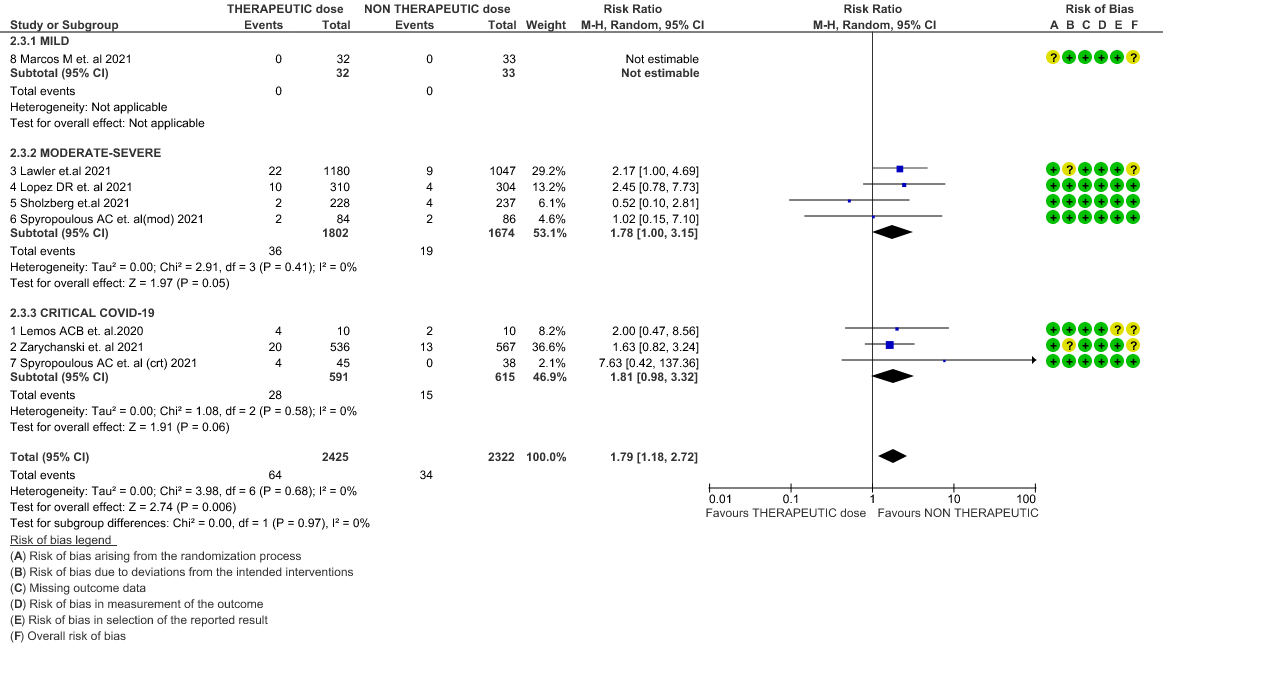
7. Composite outcome of Thrombosis or Death
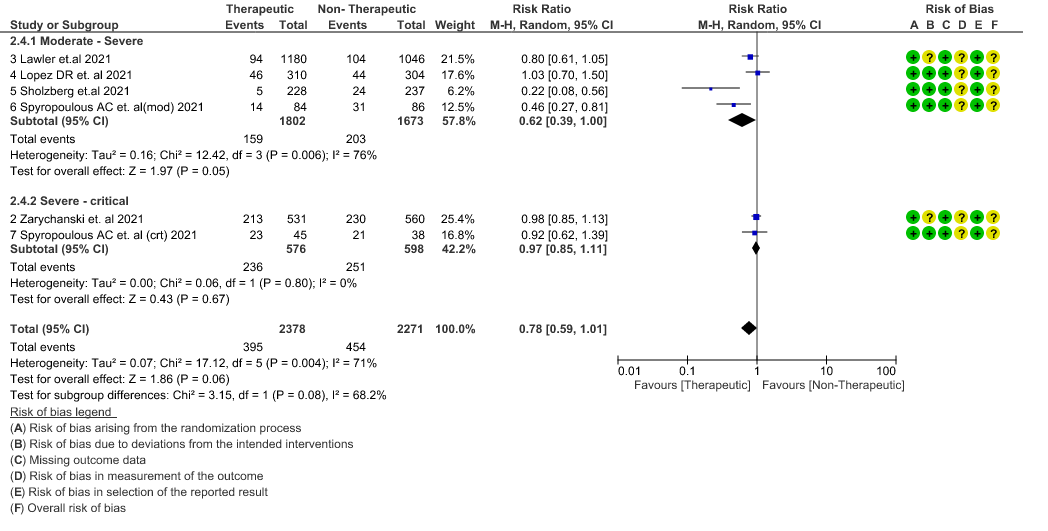
8. Organ Support free days in median (IQR)
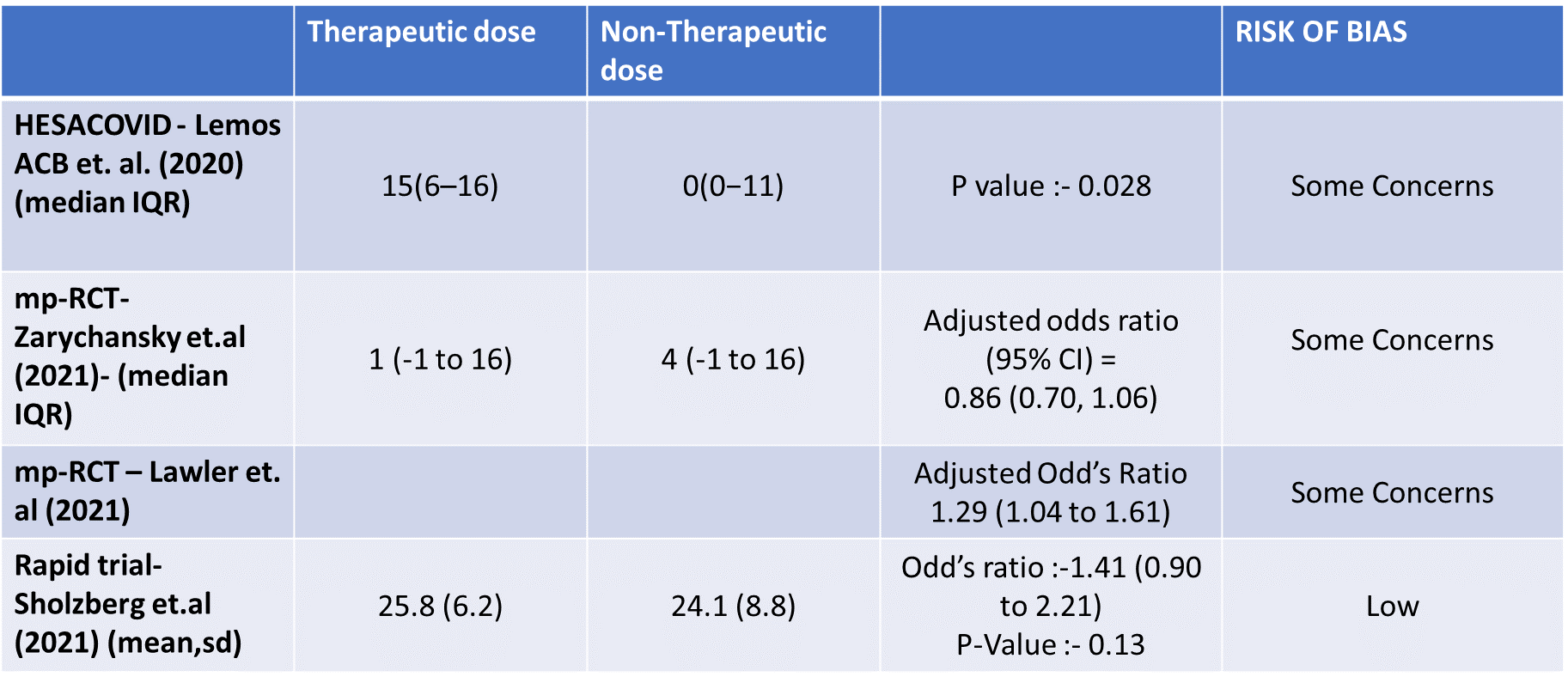
9. Composite outcome of ICU admission or Death
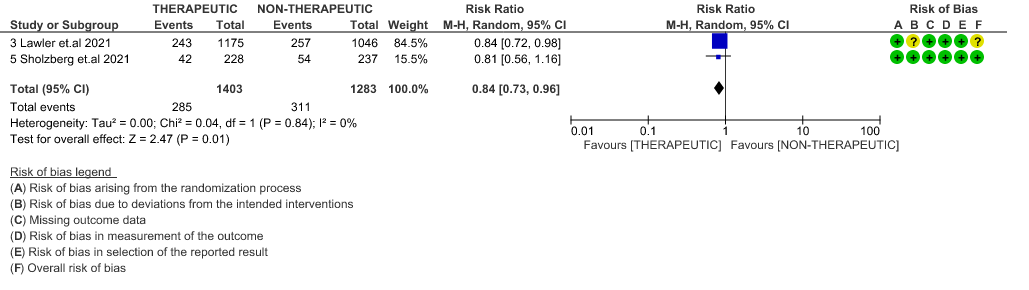
10. Survival Without Organ Support at Day 28
The Anticoagulation Expert Working Group met on 24th May 2021 to consider the use of therapeutic Vs prophylactic dose anticoagulation in the management of COVID-19. A summary and then more detailed explanations of their judgements follow.
Summary of judgements
EtD summary table
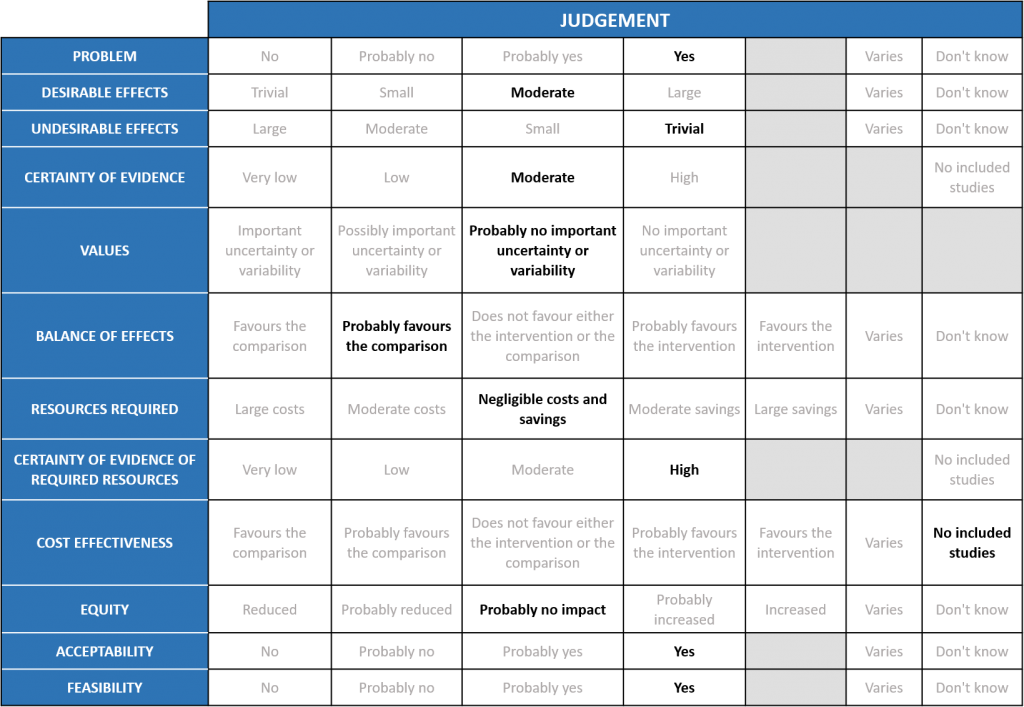
Problem
Hypercoagulability is a recognized phenomenon in COVID19 and is believed to be multifactorial. SARS-CoV-2 virus both directly and indirectly causes endothelial injury, microvascular inflammation, endothelial exocytosis, eventually contributing to an acute respiratory distress syndrome. In post-mortem studies microvascular occlusion with platelet-fibrin thrombi have been reported. In addition, changes in circulating prothrombotic factors and stasis due to immobility encountered in the critically ill has led to a recognized prothrombotic state in COVID-19 infection, translating to increased arterial and venous thrombosis. This has led to the practice of prophylactic anticoagulation for all hospitalized COVID-19 patients. However there exists an area of equipoise whether patients with severe or critical COVID19 infection will benefit with therapeutic (full dose) anticoagulation in the absence of a confirmed thrombotic event.
Desirable effects
WHO Critical: Evidence shows that using therapeutic anticoagulation did not significantly reduce mortality in critical COVID-19 but does appear to prevent thrombotic events by 35% (8-54%) with no statistically significant increase in bleeding with a moderate certainty of evidence. Therapeutic anticoagulation also did not improve days free of organ support compared to prophylactic anticoagulation. In MPRCT trial, DSMB stopped recruitment in this category as it felt that therapeutic anticoagulation did not offer any advantage towards organ support free days OSFD (as a pre specified Bayesian post probability of futility was achieved). The group discussed this at length and felt that, preventing thrombosis was important even in the absence of mortality benefit.
WHO Moderate/Severe: The group noted that using therapeutic anticoagulation reduced thrombosis and increased the probability of OSFD but did not significantly reduce mortality or cause increased bleeding in moderate/severe category of COVID-19. The group felt that thrombosis was an important event to prevent as it was difficult to recognize or confirm and probably would contribute to significant morbidity. However, the group felt that bleeding was easy to pick up and was rarely fatal. Hence the group felt that in an intensive care setting, prevention of thrombosis was an important intervention. The various thrombotic events were reviewed, and pulmonary embolism seemed to contribute to most of the events with other events recorded being myocardial infarction and strokes.
WHO mild: The evidence for therapeutic anticoagulation in mild covid-19 infection is very uncertain and the group concluded that PDA should be considered based only if the patient’s risk factor profile, necessitates it.
Undesirable effects
WHO Critical: The mPRCT (Critical) had around >500 patients in each arm and the incidence of bleeding in each group was similar. The actual difference in bleeding between the two groups was 1% suggesting that present evidence showed that the risk of bleeding was not increased. The DSMB of ACTIV-4 component of the mPRCT trial had recommended an early interim analysis to watch for the same, however the study was terminated as the futility threshold was reached suggesting that there was no benefit of TDA over PDA in the primary outcome of OSFD.
WHO Moderate/Severe: The mPRCT (Noncritical) had around >1000 patients in each arm and the incidence of bleeding in the therapeutic dose group was slightly increased suggesting that in WHO moderate and severe COVID infection there seemed to be a mildly increased risk of bleeding.
The panel also discussed regarding the absence of data regarding anticoagulation in the mild and moderate groups without hypoxia and felt that decision re anticoagulation in those groups could be based on evidence from non-randomized studies and clinical experience.
Certainty of evidence
Overall, the quality of evidence in the WHO moderate, severe and critical categories was felt to be High.
Values
The primary premise was to compare therapeutic dose anticoagulation vs nontherapeutic dose of anticoagulation in the management of hospitalized COVID-19 infection and hence clinically relevant outcomes were carefully chosen which could help a clinician on the ground level to decide which dose would of utility. The group felt that the outcomes of mortality, thrombotic events, bleeding, and Organ support free days were certainly important. They felt that there was no uncertainty or variability. The group felt that the composite outcomes of thrombosis or death and ICU admission or death in 28 days were fair outcomes that could clearly demonstrate the utility of the intervention (therapeutic dose of anticoagulation) in preventing progression of COVID-19 illness.
Balance of effects
WHO Critical: There are only 7 trials to inform evidence and there seemed to be no mortality benefit or decrease in Organ support free days [OSFD] between therapeutic anticoagulation and prophylactic anticoagulation. There was however a decrease in thrombotic events but with a possible chance of increased bleeding noted.
WHO Moderate/Severe: Therapeutic anticoagulation appeared to increase OSFD, prevent thrombotic events and a composite outcome thrombosis or death, with a slightly increased risk for major bleeding. There was no overall mortality benefit. The group felt that OSFD should be given importance in the moderate category as it saves resources and prevents morbidity.
Resource requirements
There are likely to be negligible savings or costs pertaining to implementation of the therapeutic anticoagulation. Drugs used for this are relatively cheap, widely available, and unlikely to incur a significant cost nor are they likely to result in significant savings. Health care workers in the country are experienced with the use of the same and are aware of the various monitoring implications. Cost of implementation is low and needs to weigh against hospitalization and ICU care costs. Though monitoring of anticoagulation efficacy with Anti Xa testing will be possible only in an advanced haemostasis laboratory, if widely employed to ensure therapeutic efficacy it will likely also protect against unnecessary bleeding. However, this will increase costs and is probably unnecessary in most instances. There is also possible ineffectiveness of therapy in view of high rates of heparin resistance documented in published data but overall costs of implementing therapeutic anticoagulation are likely low if we can save on costs of hospitalisation and intensive care beds.
Certainty of evidence of required resources
Resources required for implementation of therapeutic anticoagulation are minimal and the certainty of evidence for this is high.
Cost effectiveness
Now there is no data regard to the cost effectiveness of this intervention and studies need to be done to be sure of the same. The group recognized the minimal costs and resources required to deliver as it is a subcutaneous injection which can be delivered by most HCWs easily. It is also likely that even if patients in the moderate category are given therapeutic anticoagulation, hospitalization will be dictated by disease severity rather than administration of anticoagulation, given that most health care settings and health professionals are comfortable with delivery of this intervention in the outpatient settings. There are also many novel oral anticoagulants available which have been proven to have similar efficacy as heparin and could potentially be employed as substitutes, however data with these are scarce in the COVID-19 setting.
Equity
As the cost and requirements of anticoagulation delivery are reasonable, implementation can be equitable.
Feasibility
TDA is a feasible intervention which can be easily implemented in all health care settings by any health care professional.
As the Omicron variant has taken over as the variant of concern over the delta variant, the evidence for use of therapeutic anticoagulation in mild COVID-19 patients is uncertain and use of anticoagulation in out-patients is out of the scope of this review.
The working group felt that therapeutic anticoagulation in hospitalized patients with moderate disease (those who have progressively increasing O2 requirement) or severe disease might prevent thrombotic events, without any reduction in mortality. This subset might have increased probability of surviving to hospital discharge with reduced need for ICU-level organ support, including invasive and non-invasive mechanical ventilation (OSFD), with slightly increased risk for major bleeding. The evidence in critical group shows some benefit but the group felt it should be a case-to-case decision based on clinical judgement since in critical patients, bleeding maybe catastrophic. The trials did not show much difference in organ support free days in both the therapeutic anticoagulation and prophylactic anticoagulation groups.
Though there was no data regard to the cost effectiveness of this intervention, resources required for implementation of therapeutic anticoagulation are minimal in this subgroup of patients. This intervention of therapeutic dose anticoagulation is feasible to implement widely and easily. The evidence is for injectable low molecular weight heparin, unfractionated heparin, and oral anticoagulants.
The subgroups of children <18 years of age, pregnant women, and asymptomatic were excluded from most studies. Some studies also excluded those on dialysis for chronic kidney disease, chronic liver, and lung diseases, as well as those on antiplatelet therapy.
Mild and non-severe COVID patients were evaluated in one RCT. The BEMICOP 1study included only non-severe COVID patients and compared prophylactic vs therapeutic doses of Bemiparin. This study favoured a prophylactic dose of Bemiparin. However, the study was limited by its small sample size (32 in the therapeutic arm and 33 in the prophylactic arm). Anticoagulation should be avoided for outpatient mild COVID cases even though this subgroup of patients has not been studied in RCT.
There is no defined role for monitoring of D-dimer in non-hospitalized COVID-19 patients. A high D-dimer is known to be associated with an increased risk of venous thromboembolism in patients without COVID-19. In COVID-19, D-dimer has been correlated with a poorer prognosis and increased mortality. However, whether a high D-dimer value can be extrapolated to an increased risk of arterial or venous thrombosis in COVID-19, however it has been associated independently with a higher risk of mortality 19. Hence in hospitalized patients with COVID-19 with no clinical or radiological evidence of thrombosis, there is insufficient data to recommend regular monitoring of D-dimer or base management strategies on the same.
For practical purposes, especially as far as decision regarding the role of therapeutic anticoagulation is concerned, we suggest continuing the previous WHO classification of the disease spectrum into mild, moderate, severe and critical categories20.
Data pertaining to the role of anticoagulation in mild category of disease is sparse and needs to be covered separately since it was not a part of this PICO Question, and these patients now are often managed as out-patients. There is a need for monitoring this arm though and we propose an independent evaluation of this subset of patients during the coming waves, if any.
For patients with moderate disease with hypoxia and for those with severe disease, the balance of effects comparing the risk of thrombosis vs risk of bleeding continues to favor therapeutic dose anticoagulation over prophylactic dose anticoagulation. These seemed to be a significant difference in the number of patients falling into these two groups in the third wave as compared to the second wave i.e., with more patients with mild as compared to severe or critical disease all over the country.
For patient with critical disease, though the data is not clear in this category, since the worsening from severe to critical disease often proceeds as a continuum, it would be practically difficult to switch from therapeutic to prophylactic modality in a given patient unless clear criteria for stopping anticoagulants are met. Bleed scores may be used in this regard. More research is therefore needed in this area.
A preponderance of observational, non-randomized data suggests increased risk of thrombosis in all severities of COVID-19, correlation of D-Dimer with disease severity and benefit of post-discharge anticoagulation. These findings are not borne out in randomized trials and hence will not be considered in the framing of these guidelines. Therefore, we need to be cautious towards interpretation of any data relating to outcomes with different dosing strategies, as this would be non-randomized data.
1.Research needs to focus on duration of anticoagulation in COVID-19 in critical and severe cases.
2. Role of D dimer in tailoring anticoagulation
3. Role of Anticoagulation in COVID-19 as prophylaxis in high-risk groups with mild COVID 19.
4. Anticoagulation with varying clinical picture as seen with different variants of COVID-19
5. Role of anticoagulants in Vaccine Induced Thrombotic Thrombocytopenia.
6. Duration of anticoagulants in Vaccine Induced Thrombotic Thrombocytopenia
- Marcos-Jubilar, M. et al. Therapeutic versus Prophylactic Bemiparin in Hospitalized Patients with Nonsevere COVID-19 Pneumonia (BEMICOP Study): An Open-Label, Multicenter, Randomized, Controlled Trial. Thromb. Haemost. 122, 295–299 (2022).
- Lopes, R. D. et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. The Lancet 397, 2253–2263 (2021).
- Lemos, A. C. B. et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 196, 359–366 (2020).
- Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N. Engl. J. Med. 385, 777–789 (2021).
- Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N. Engl. J. Med. 385, 790–802 (2021).
- Sholzberg, M. et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 375, n2400 (2021).
- Spyropoulos, A. C. et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 181, 1612–1620 (2021).
- Wang, D. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
- Tang, N., Li, D., Wang, X. & Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. JTH 18, 844–847 (2020).
- Klok, F. A. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 191, 145–147 (2020).
- Ackermann, M. et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128 (2020).
- Hoffmann, M. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271-280.e8 (2020).
- Cuker, A. et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: January 2022 update on the use of therapeutic-intensity anticoagulation in acutely ill patients. Blood Adv. bloodadvances.2022007561 (2022) doi:10.1182/bloodadvances.2022007561.
- Moores, L. K. et al. Thromboprophylaxis in Patients With COVID-19: A Brief Update to the CHEST Guideline and Expert Panel Report. CHEST 0, (2022).
- Morici, N. et al. Enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients: comparison of 40 mg o.d. vs 40 mg b.i.d. The X-COVID19 Randomized Clinical Trial. http://medrxiv.org/lookup/doi/10.1101/2021.11.17.21266488 (2021) doi:10.1101/2021.11.17.21266488.
- INSPIRATION Investigators et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 325, 1620–1630 (2021).
- Perepu, U. et al. Standard Prophylactic Versus Intermediate Dose Enoxaparin in Adults with Severe COVID-19: A Multi-Center, Open-Label, Randomised Controlled Trial. https://papers.ssrn.com/abstract=3840099 (2021).
- Connors, J. M. et al. Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA 326, 1703–1712 (2021).
- Li, Y. et al. Clinical Significance of Plasma D-Dimer in COVID-19 Mortality. Front. Med. 8, 638097 (2021).
- Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1.
Covid Management Guidelines India Group - Anticoagulation Working Group. Prophylactic Vs Therapeutic dose anticoagulation. Covid Guidelines India; Published online on August 08th, 2022; URL: https://indiacovidguidelines.org/therapeutic-dose-vs-nontherapeutic-dose-of-thromboprophylaxis/(accessed <date>)
Therapeutic dose Vs Nontherapeutic dose of thromboprophylaxis – Previous Recommendation
